
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
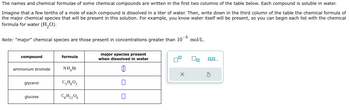
Transcribed Image Text:The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of
the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical
formula for water (H₂O).
Note: "major" chemical species are those present in concentrations greater than 10-6 mol/L.
compound
ammonium bromide
glycerol
glucose
formula
NH₂Br
C₂H₂O3
C6H₁2O6
major species present
when dissolved in water
00
0
X
0.0....
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 compound sodium cyanide copper(II) sulfate nickel (II) chloride formula NaCN CuSO4 NiCl₂ major species present when dissolved in water mol/L.arrow_forwardneed help with this chemistryarrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water ( H2O ). Note: "major" chemical species are those present in concentrations greater than 10−6/molL . compound formula major species presentwhen dissolved in water acetone CH32CO ? isopropanol C3H8O ? nitrous oxide N2O ?arrow_forward
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 compound sucrose fructose magnesium sulfate formula C₁2H22011 C6H₁2O6 MgSO4 major species present when dissolved in water U 7 X mol/L.arrow_forward7. For a chemical analysis, 750 mL of a 0.480 mol/L potassium permanganate solution is to be prepared. Calculate the mass of potassium permanganate crystals that will be dissolved to make this solution. d) Basoarrow_forwardA chemist samples a river's water to measure the amount of fertilizer runoff from the area farms. In a 0.0740 L sample, the chemist measures 562 µg of nitrate. Express the concentration of nitrate in parts per million (ppm) and parts per billion (ppb). Assume the density of the river water sample is 1.00 g/mL. concentration: concentration: ppm ppbarrow_forward
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: "major" chemical species are those present in concentrations greater than 10 mol/L. major species present when dissolved in water compound formula 0,0,.. iron(II) bromide FeBr, sodium nitrate NaNO3 nitrous oxide N,0arrow_forwardMost biological reactions take place in an aqueous (water-filled) environment. For example, salts like NaCl readily dissolve in water. However, NaCl is not soluble in a solvent like benzene. Based on what you know about the chemistry of water, explain why this is the case.arrow_forwardThe density of a solution that is 20.0% HClO4 is 1.138 g/ml. Calclulate the molarity of the HClO4 (molar mass is 100.46 g) solutionarrow_forward
- A salt has a molar solubility of 4.18 M at 21.0°C. What is the the maximum amount of salt (in moles) that can dissolve in 1.495 L of solution before the solution becomes supersaturated at 21.0°C? Assume the salt has a molar mass of 55.44 g/mol. Report your answer to 2 decimal places.arrow_forward! T 1 A Z The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 mol/L. 89 FI compound propylene glycol Explanation ammonium lodide @ 2 glycerol W S X command Check 30: F2 # 3 formula C,H, (OH)₂ C₂H₂O, NH₂L 20 E E D 8.0 F3 C $ 4 R major species present when dissolved in water F a F4 % 5 V 0 0 T I F5 G 6 B F6 Y 7° H X & 7 00 stv N K F7 U 0.0.... 3 J © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A- alt ✓ A A C 8 DII FB 1 M ( 9 K F9 O <…arrow_forwardSodium carbonate dissociates when it dissolves in water as follows: If the concentration of the sodium ions is 0.817 mol/L, then what is the concentration of the sodium carbonate solution? Record only your numerical answer with the correct number of significant digits. You do not need to include units as the units appear for you beside the answer box already.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY