
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:!
T
1
A
Z
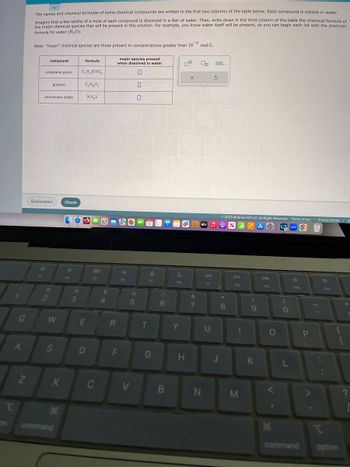
The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of
the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical
formula for water (H₂O).
-6
Note: "major" chemical species are those present in concentrations greater than 10 mol/L.
89
FI
compound
propylene glycol
Explanation
ammonium lodide
@
2
glycerol
W
S
X
command
Check
30:
F2
#
3
formula
C,H, (OH)₂
C₂H₂O,
NH₂L
20 E
E
D
8.0
F3
C
$
4
R
major species present
when dissolved in water
F
a
F4
%
5
V
0
0
T
I
F5
G
6
B
F6
Y
7°
H
X
&
7
00
stv
N
K
F7
U
0.0....
3
J
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A-
alt ✓ A
A
C
8
DII
FB
1
M
(
9
K
F9
O
<
)
0
L
200
F10
command
P
F11
{
option
+
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution contains 6.79 g of K₂Cr₂O, per 100 g of water at 30 °C. Use the solubility graph to determine if the K₂Cr₂07 solution is saturated, unsaturated or supersaturated. supersaturated saturated unsaturated A solution contains 88.3 g of Pb(NO3)2 per 100 g of water at 10 °C. Use the solubility graph to determine if the Pb(NO3)2 solution is saturated, unsaturated or supersaturated. unsaturated saturated supersaturated Solubility (g solute in 100 g H,O) 100 90- 80- 70- 60- 50- 40 30 20- 10- 0- 0 CaCl₂ NaNO, KNO, 10 20 30 40 Na,SO, 50 60 Temperature (°C) Pb(NO,), KCIO, 70 80 K₂Cr₂O KCI NaCI 90 100arrow_forward12.2 g of isopropyl alcohol is added to 314.7 mL of water. Find the concentration of this solution in units of volume percent. The density of isopropyl alcohol is 0.786 g/mL.arrow_forwardCan you please answer this and give a explanation if possible?arrow_forward
- An aqueous solution is made by dissolving 14.5 grams of manganese(II) sulfate in 463.8 grams of water. Calculate the molality of manganese(II) sulfate in the solution. Note: Enter your answer to 3 decimal places. DO NOT write in the units. DO NOT use scientific notation.arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: "major" chemical species are those present in concentrations greater than 10 9- mol/L. major species present when dissolved in water compound formula O,0,. propylene glycol C;H, (OH), methanol CH, OH nickel(II) bromide NiBr,arrow_forwardThink of different instances from everyday life that involve one or more substances (solutes) dissolving in water. What can your observations tell you about the type of solution formed? Is it a strong electrolyte, weak electrolyte, or nonelectrolyte solution? How do the properties of the solution differ from that of a pure solvent? Include a list of references.arrow_forward
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H2O). Note: "major" chemical species are those present in concentrations greater than 10^−6/molL . compound formula major species presentwhen dissolved in water copper(II) chloride CuCl2 propylene glycol C3H6OH2 sucrose C12H22O11arrow_forwardCan you please answer this and show your steps?arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 compound sodium cyanide copper(II) sulfate nickel (II) chloride formula NaCN CuSO4 NiCl₂ major species present when dissolved in water mol/L.arrow_forward
- need help with this chemistryarrow_forwardPredicting the product The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). Note: "major" chemical species are those present in concentrations greater than 10 -6 compound potassium hydroxide iron(11) iodide iron(II) sulfate Explanation Check formula KOH Fel₂ FeSO4 major species present when dissolved in water 0 0 0 X mol/L. 0.0.... S Graw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessitarrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water ( H2O ). Note: "major" chemical species are those present in concentrations greater than 10−6/molL . compound formula major species presentwhen dissolved in water acetone CH32CO ? isopropanol C3H8O ? nitrous oxide N2O ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY