
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
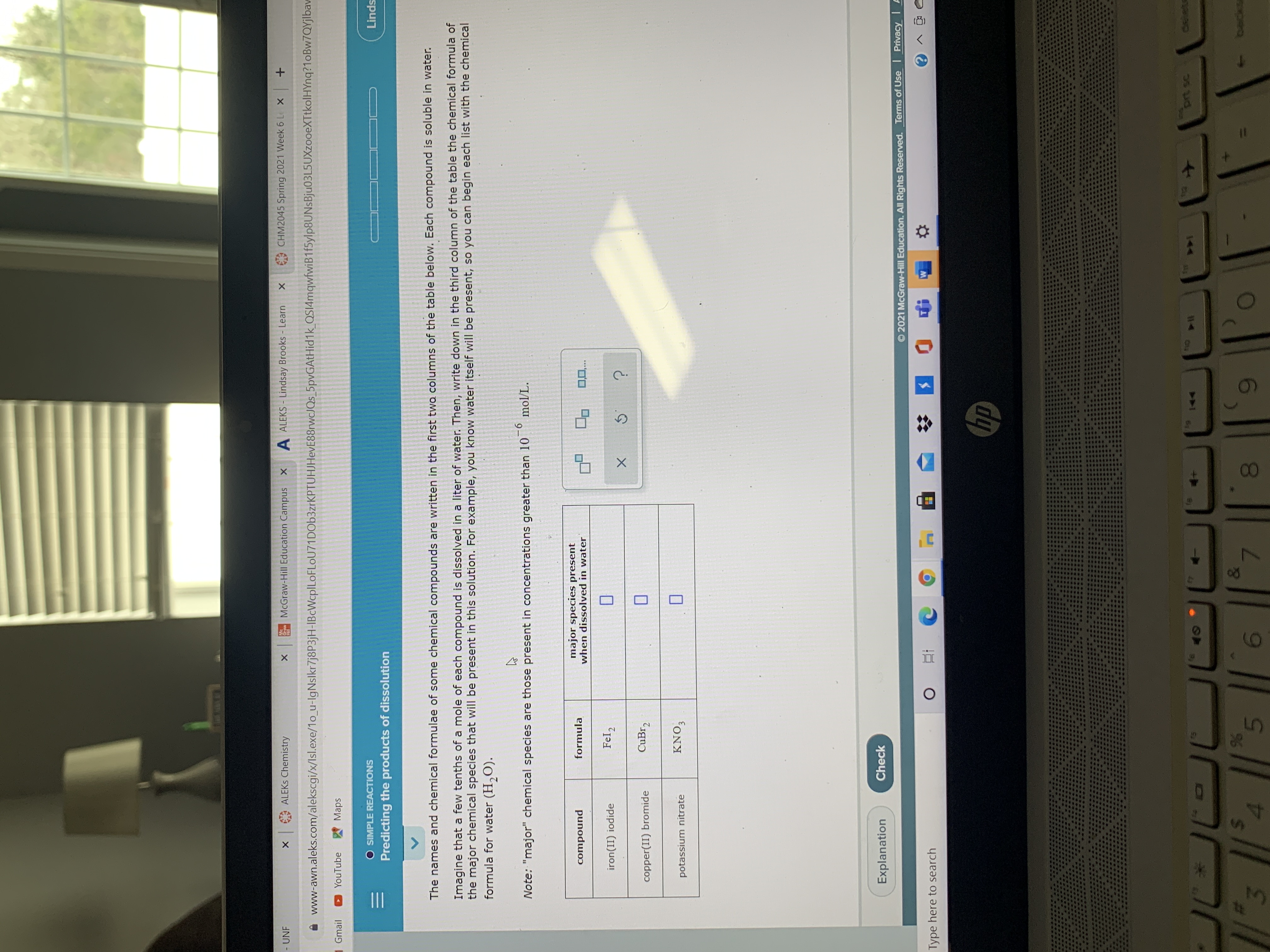
Transcribed Image Text:The names and chemical formulae of some chemical compounds are written in the first twa columns of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of
the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical
formula for water (H,O).
Expert Solution
arrow_forward
Step 1
Iconic compounds are easily soluble in water and they can dissociate into respective ions in solution.
Metals bind with non metals to form mostly ionic compounds.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following phrases best describes the term solubility? the ability of a solvent to dissolve in a solute the ability of a solute to dissolve in a solvent the maximum amount of solute that will dissolve in solvent at a given temperature the maximum amount of solvent that will dissolve in a solute at a given temperature the amount of solvent that dissolves in a solute for each 10oC rise in temperaturearrow_forwardA salt has a molar solubility of 4.18 M at 21.0°C. What is the the maximum amount of salt (in moles) that can dissolve in 1.495 L of solution before the solution becomes supersaturated at 21.0°C? Assume the salt has a molar mass of 55.44 g/mol. Report your answer to 2 decimal places.arrow_forward! T 1 A Z The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 mol/L. 89 FI compound propylene glycol Explanation ammonium lodide @ 2 glycerol W S X command Check 30: F2 # 3 formula C,H, (OH)₂ C₂H₂O, NH₂L 20 E E D 8.0 F3 C $ 4 R major species present when dissolved in water F a F4 % 5 V 0 0 T I F5 G 6 B F6 Y 7° H X & 7 00 stv N K F7 U 0.0.... 3 J © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A- alt ✓ A A C 8 DII FB 1 M ( 9 K F9 O <…arrow_forward
- A student dissolves 1.9 g of styrene (C,H,) in 150. mL of a solvent with a density of 1.02 g/mL. The student notices that the volume of the solvent does not change when the styrene dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits. molarity = x10 molality 미□arrow_forwardThis question is part two of three. When answering this problem, report the answer with the appropriate number of significant figures. When entering units, use proper abbreviated units with proper capitalization. A solution is prepared by combining 5.92 grams of an unknown non-electrolyte with 240.0 grams of chloroform. The freezing point of the mixture is -64.80 oC, while the freezing point of pure chloroform is -63.50oC. The kf of pure chloroform is 4.68oC/molal. Based on this information, how many moles of solute are present in this solution? What is the molar mass of the solute? Report both answers in scientific notation with appropriate sig figs.arrow_forwardA solid consists of a mixture of NaNO3 and Mg(NO3)2. When 6.50 g of the solid is dissolved in 50.0 g of water, the freezing point of the solution is lowered by 5.28°C. What is the composition by mass of the solid? g NaNO3 g Mg(NO3)2arrow_forward
- The solubility of CuBr in water at 25 °C is measured to be 0.010 L Round your answer to 2 significant digits. 0 Dx1 x10 Ś Use this information to calculate K for CuBr. sparrow_forwardWhat are the similarities and differences between the everyday and technical meanings and uses of the terms insoluble and solubility ?arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 compound sodium cyanide copper(II) sulfate nickel (II) chloride formula NaCN CuSO4 NiCl₂ major species present when dissolved in water 0 X mol/L. Śarrow_forward
- A student dissolves 17. g of sucrose (c,H,„0,,) in 250. mL of a solvent with a density of 1.04 g/mL. The student notices that the volume of the solvent does not change when the sucrose dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits. molarity molalityarrow_forwardYou are to prepare about 1 L of a solution which contains approximately 0.100 mole NaOH per liter. Before you begin, you should calculate the amount of saturated NaOH you will need for this solution. Assume that the saturated NaOH is approximately 3.5 M. Since the number of moles of NaOH will not change upon dilution, your calculation should be of this form: mol NaOH before dilution = mol NaOH after dilution mol NaOH before dilution = 3.5 M x ?? L mol NaOH after dilution = 0.100 M X ?? L ?? L (saturted NaOH) = (0.100 M x 1 L) / (3.5 M)arrow_forwardA student dissolves 8.0 g of phenol (CH₂OH) in 125. mL of a solvent with a density of 1.06 g/mL. The student notices that the volume of the solvent does not change when the phenol dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits. molarity molality = x10 DO 0x0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY