
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Plz do Asap...!
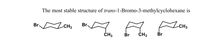
Transcribed Image Text:The most stable structure of trans-1-Bromo-3-methylcyclohexane is
ICHS
Br.
Br.
-CH3
ČH3
Br
ČH3
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- to make a tablet of aspirin, it requires 249 mg of salicylic acid. if the chemist purchased 9.0 x 10^-3 OZ of salicylic acid, are these enough to make one table of aspirin?arrow_forwardAsk Laftan Anlamaz - Episode St. John's University - My App X A ALEKS - Iffat Khan - Learn G In valence electrc -> A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLgkt1FLIq7wcPWKzBYGfE9IMFj8s1-UE-m2k5S O MATTER Finding the side length of a cube from its volume in liters A technical machinist is asked to build a cubical steel tank that will hold 390 L of water. Calculate in meters the smallest possible inside length of the tank. Round your answer to the nearest 0.01 m. Omarrow_forwardT LI ALEKS - Rafia Riaz - Knowledge CX b Answered: = Module Knowledge X + www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusZsUfYJJOecqshL9ihmdkZd4zbXDleN-ax_luBKRpjrEFpTCwvorn6Uz9RRiR?1... Q (297) Yeh scene kuch samajh nai X Type here to search = Module Knowledge Check For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. A few moles of nitrogen (N₂) gas. System A few moles of helium (He) gas. A few grams of ammonia vapor (NH₂). I Don't Know Et Submit Change The nitrogen is heated from -3.0 °C to 81.0 °C and is also compressed from a volume of 15.0 L to a volume of 9.0 L. The helium is compressed from a volume of 4.0 L to a volume of 3.0 L while the temperature is held constant at…arrow_forward
- Collig Notes (1).pdf A ALEKS -Jeneen Abdelra X M Mathway Algebra Prol molar mass of zinc chic x Launch Meeting-Zoom x G 0.16kg to g-Google Se x + www-awu.aleks.com/alekscgi/x/Isl.ex Mathway | Algebra Problem Solver WoxT77ErtzZpGbzESWMk5SbMzyGfsmkWQSq2n2qsprma6JcCeltVTwZDkOLh51Rytx20V.. * mathway.com O GASES, LIQUIDS, AND SOLIDS Using the Kf and Kb equations with electrolytes Jeneen v The normal boiling point of a certain liquid X is 148.70 °C, but when 13. g of alanine (C,H,NO,) are dissolved In 250.g of X the solution bolls at 149.5 °C İnstead. Use this information to calculate the molal boiling point elevation constant K, of X. Be sure your answer is rounded to the correct number of significiant digits. alo °C kg K, = 0 mol x10 9. Check Explanation Privacy Accessibility 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use 5:5arrow_forwardn Course: CHEM262-SEC01 Org x V OWL Exam O Noor Abid - 32843659 S x b Success Confirmation of Quest x G how to take a screenshot on m + A owl.umass.edu/owlj/servlet/Student Update : M Gmail O YouTube O Google Keep n UMass Amherst M.. c Write The Comple... O SPIRE Logon A Swank Movies O Discovering Nutrit. 26 UMass Amherst -. t. Minitab >> Select the major product of the following reactions. 1. NaOEt 2. CH3I EtO OEt 3. NaOEt 4. CH3I 5. H,0*/ heat Is 6. CH,ОН/ Н+ HO, OH IV I II III O None of the choices O IV O IIarrow_forward4. Number these in order of ease of hydrolysis. 1 = easiest to hydrolyze, 4 = hardest to hydrolyze. C.H.&CI C6H5CNHC(CH3)3 CH₂C=NCHCOCHarrow_forward
- 12: Mastery - Kinetics X com/ilm/takeAssignment/takeCovalentActivity.do?locator=assignment-take juju_ ncepts " Q Search OWLv2 | Online teaching and le Free Online Survey... [CO] = [Cl₂] = [COCI₂] = Submit Answer Home - Academia... PRO Modules Create Your Rubric-... [Review Topics] [References] Use the References to access important values if needed for this question. The equilibrium constant, K, for the following reaction is 77.5 at 600 K. CO(g) + Cl₂(g) CoCl₂(g) Calculate the equilibrium concentrations of reactant and products when 0.474 moles of CO and 0.474 moles of Cl₂ are introduced into a 1.00 L vessel at 600 K. Retry Entire Group IL & M M M X + hp 4+ 7 more group attempts remaining 8 19 ( 9 110 The Utility Experts ... m 4 12 = insert » | ← 12/1 baarrow_forward● A ALEKS-Lara AX → с www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIntrzjVfiaJ_cKvcyqWTUEIAKXtcoqhtE9_vix... 4 Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of.... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... C ! 1 Under these conditions, calculate the reaction free energy AG for the following chemical reaction: N₂(g) + 3H₂(g)2NH₂(g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. ● ENTROPY AND FREE ENERGY Calculating reaction free energy under nonstandard conditions Explanation A T A chemist fills a reaction vessel with 7.76 atm nitrogen (N₂) gas, 7.51 atm hydrogen (H₂) gas, and 0.524 atm ammonia (NH₂) gas at a temperature of 25.0°C N Puppy Jobs, Em x G puppy store ne x Part Time Jobs, x G Food Server and X G 10 out of 25 as x) How Many Hour x CO @ 2 Check W S X R option command #3 X E D 26 $ DS 4 C Ś R LL F % 5 V T tv SH N G MacBook Pro ^ 6 Y B & M 7 19.6 Reduction…arrow_forwardMALEKS T LI ALEKS - Rafia Riaz - Knowledge CX + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtHPKeXbFh_esrw9lm-BbWISQqFfd1OKKzQ55nmjVf-6W1kBQ5aycVAYBaQo?... Type here to search = Module Knowledge Check Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. A chemical reaction between two liquids creates gaseous products. A gas expands, absorbing heat from its surroundings. Change During an exothermic chemical reaction, a solid is consumed and a gas produced. I Don't Know Et Submit Question 1 3 Is this change spontaneous? Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. X Rafia V ? 13 ollo © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 65°F Mostly clear (?) F * (P 9:08 PM 5/10/2023 x : 6arrow_forward
- me File Edit View History Bookmarks Profiles Tab Window Help ●● → C ALEKS ALEKS - Tiffany Te-L-Peng.- L X T My Courses - Temple Universi X www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liGBdp5ulp5VqUnMDyPOVaX6q0CRPLZSQ5Ne_4EfX10krk = O ELECTROPHILIC ADDITION REACTIONS Recognizing common alkene addition reactions X Classify each of the following organic reactions. Explanation Check reaction 1) Hg(OAc)₂, H₂O 2) NaBH HBr ROOR H₂ ~=~ Pd Cl₂ Br CI CI B OH type of reaction (check all that apply) inhalation hydrohalogenation addition Ohydration Odihydroxylation halohydrin formation addition Odihydroxylation Ohydration halohydrin formation substitution hydrohalogenation O halohydrin formation subtraction addition Ohydration Odihydroxylation Ohydrohalogenation halohydrin formation Oenolation Ohydration Ohydrohalogenation O addition Odihydroxylation + Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Term MacBook Proarrow_forwardALEKS - Jeneen Abdelrahim - Lea x A www-awu.aleks.com/alekscgi/x/Isl.exe/1o u-IgNslkr7j8P3jH-lix5uFZIVj2iEJjQd1WoxT77ErtzZpGbzESWCHQAIM8yRRgXuzfCF2uqajotou CbXDqDRhYOu6ecw75TbH3KAJL.. O STOICHIOMETRY Solving for a reactant using a chemical equation Jen Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the air to form water (H,0) and acetic acid (CH,COOH), the main ingredient of vinegar. What mass of ethanol is consumed by the reaction of 5.1 g of oxygen gas? Be sure your answer has the correct number of significant digits. Privacy Check 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use Explanation 三Iarrow_forwardMALEKS T LI ALEKS - Rafia Riaz - Knowledge CX + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtHPKeXbFh_esrw9lm-BbWISQqFfd1OKKzQ55nmjVf-6W1kBQ5aycVAYBaQo?... Type here to search = Module Knowledge Check A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 33.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY