
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
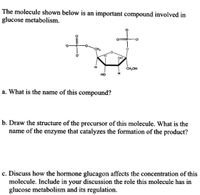
Transcribed Image Text:The molecule shown below is an important compound involved in
glucose metabolism.
CH2
OH
CH,OH
но
a. What is the name of this compound?
b. Draw the structure of the precursor of this molecule. What is the
name of the enzyme that catalyzes the formation of the product?
c. Discuss how the hormone glucagon affects the concentration of this
molecule. Include in your discussion the role this molecule has in
glucose metabolism and its regulation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Another antidote for methanol poisoning is fomepizole, which is an aldehyde dehydrogenase (ADH) inhibitor. Would fomepizole be more or less effective than ethanol? Explain your reasoning.arrow_forwardAll the Bold answers are wrong please explain why it is wrong and give me the correct answer. Thanks, in advanced Which of the following is true about enzymes? Enzymes are equally effective across broad ranges of temperature and Ph. Enzymes lower the overall free energy of a reaction to make it spontaneous. Enzyme activity is generally unregulated. Enzymes recognize many substrates with equal specificity. The insulin receptor catalyzes the phosphorylation of several substrates and is therefore classified as a Transferase Phosphate Kinase Lyase Oxidoreductase 3)Which of the following reaction parameters can enzymes optimize to increase reaction rate? The proximity(=closeness) of the reacting groups. The rotational motions of the substrates and catalytic groups. The orientations of the substrates and catalytic groups. The achieve energy needed to achieve the transition state. The catalytic mechanism of RNA relies upon general acid-base catalysis involving the amino acid…arrow_forwardIntravenous injection of KCl could be fatal. Why?arrow_forward
- Fill in the blanks and answer the question: A. The enzyme ATCase (aspartate transcarbamoylase) uses _________________control to regulate its activity. The molecule ATP is an allosteric _____________ of ATCase and because it is a molecule different from the substrate of this enzyme it produces an _________________ effect in the enzyme’s activity. B. Is it appropriate to study the enzymatic activity of ATCase with the Michaelis Menten approximation? Justify your answer.arrow_forwardThe Krebs cycle reaction shown below is catalyzed by __ enzyme and ___ pays for this reaction note only major metabolites are shown a. Synthetase, hydrolysis of acetyl CoA b. Synthase, hydrolysis of acetyl CoA c. Synthase, hydrolysis of NADH d. Synthetase, hydrolysis of ATParrow_forwardThe following molecules act as either inhibitors or activators of the enzyme that converts fructose-6-phosphate to fructose-1,6- diphosphate. Which do you think are activators? (A) ADP B AMP ATP citratearrow_forward
- Draw the products that would be produced from phospholipase C cleavage of a glycerophospholipid with a phosphoserine head group, an alpha linoleic acid attached to C2 of glycerol and a linoleic acid attached to C, of glycerol. Draw these products at pH=1. Be sure to number the fatty acid carbons in your products.arrow_forwardChoose the best answers for each missing word from the list below. ____ regulated by ATP, Aspartate transcarbamoylase (ATCase) is (1) which is a (2)________________ regulator, and by CTP which binds to the (3)____ subunit of ATCase and shifts the conformational equilibrium to the (4). T state. CTP regulation of ATCase is called (5)________ 1) allosterically, 2) positive, 3) regulatory, 4) inactive, 5) feedback inhibition 1) covalently 2) positive, 3) regulatory, 4) active, 5) feedback inhibition 1) allosterically, 2) negative, 3) regulatory, 4) inactive, 5) feedforward activation 1) allosterically, 2) negative, 3) catalytic, 4) active, 5) feedforward inhibition 1) irreversibly, 2) positive, 3) catalytic, 4) inactive, 5) feedback inhihitionarrow_forwardWatch this video and answer the question below https://youtu.be/AtlCxYDxY1I BCR-ABL is a kinase. A kinase is a type of enzyme that catalyzes the transfer of phosphate from one molecule to another. What are the substrates for the reaction that BCR-ABL catalyzes? Select all that are true.a- Substrate protein b- BCR-ABL c-ATP d- None of the listed molecules herearrow_forward
- Hello, can someone please help me answer 1 and 2 while focusing on the interaction that the enzyme, the substrate and the product have. Thank youarrow_forwardThe catalytic activity of enzymes depends on the presence of appropriate environmental conditions. Pepsin is a digestive enzyme found in the stomach and facilitates the digestion of large proteins. If pepsin is removed from this acidic environment of the stomach and is instead placed in a more basic environment, it will cease to function. Describe the specific effect that a change in environmental pH will have on pepsin and explain how this change can lead to inhibition of its catalytic activity. Respond in 4 to 6 complete sentences.arrow_forwardThe enzyme phosphoglycerate mutase promotes which of the following? a. the hydrolysis of 3-phosphoglycerate b. the isomerization of 3-phosphoglycerate c. the oxidation of 3-phosphoglycerate d. the reduction of 3-phosphoglyceratearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON