
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
can you please answer the question in the picture
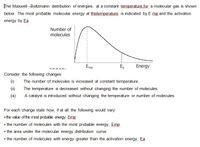
Transcribed Image Text:The Maxwell -Boltzmann distribution of energies, at a constant temperature.for a molecular gas is shown
below. The most probable molecular energy at thistemperature is indicated by E mp and the activation
energy by Ea
Number of
molecules
Emp
E,
Energy
Consider the following changes:
(1)
The number of molecules is increased at constant temperature.
(ii)
The temperature is decreased without changing the number of molecules.
A catalyst is introduced without changing the temperature or number of molecules.
For each change state how, if at all, the following would vary:
• the value of the most probable energy, Emp
• the number of molecules with the most probable energy, Emp
• the area under the molecular energy distribution curve
• the number of molecules with energy greater than the activation energy, Ea
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please solve for questions 1-8 using the given data, show your work.arrow_forwardPredict the reagent(s) needed to produce these productsarrow_forwardA pure substance that is composed of only one type of atom is______. Group of answer choices a particulate a homogeneous mixture a heterogeneous mixture an element a macroscopic particlearrow_forward
- Question 3 ) Could please explain. I've been watching videos, but none have been adding a whole new product...arrow_forwardSee the following table of concentration, green intensity, blank. Based on these data, convert intensity data into absorbance. Make a plot of absorbance versus relative concentration. Find the equation for the best fit line on the plot. Concentration (M) Green intensity (I) Blank (lo) Absorbance (-log 1/10) 0.000 234 234 0.100 228 234 0.200 221 234 0.300 216 234 0.400 211 234 0.500 203 234 0.600 199 234 0.700 191 234 0.800 185 234 0.900 179 234 1.000 174 234 a) Show the best fit line as the form of y = ax + b for your answer. You do not need to show your plot. How much is the slope (m) in this best fit line as the form of y = mx + b in your answer? Type your answer...arrow_forwardThe concentrations of K* in water were measured with atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES). Four water samples obtained at different cities were used, and each sample was measured one time. The measurements are shown in the table below: Tulare Clovis 8.9 ppm 6.7 ppm 7.0 ppm 5.0 ppm What is the calculated t-value (tcalculated) for the statistical comparison of these two methods? AAS AES 1.908 2.664 2.309 2.938 3.012 Fresno 5.5 ppm 5.4 ppm Selma 12.5 ppm 9.8 ppmarrow_forward
- How to answer number 67arrow_forwardWhat is the unit of the answer?arrow_forwardA meniscus is "read" at the bottom of the convex curve and can only be reported to one more significant figure than is labeled on the buret. Draw a meniscus on each of these burets (different volume in each one) and read the meniscus and determine the volume of the difference between the two burets.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY