
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I need all parts
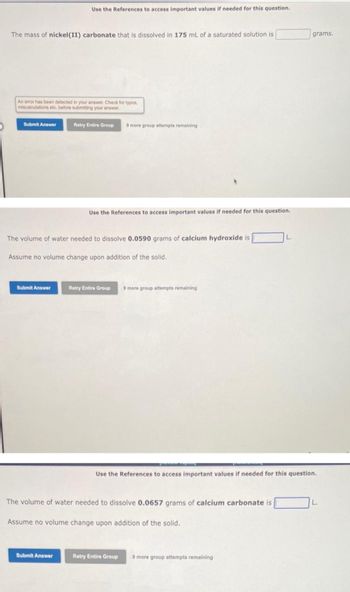
Transcribed Image Text:The mass of nickel (II) carbonate that is dissolved in 175 mL of a saturated solution is
An error has been detected in your answer. Check for typos,
miscalculations etc. before submitting your answer.
Submit Answer
Use the References to access important values if needed for this question.
Submit Answer
Retry Entire Group 9 more group attempts remaining
The volume of water needed to dissolve 0.0590 grams of calcium hydroxide is
Assume no volume change upon addition of the solid.
Submit Answer
Use the References to access important values if needed for this question.
Retry Entire Group 9 more group attempts remaining
The volume of water needed to dissolve 0.0657 grams of calcium carbonate is
Assume no volume change upon addition of the solid.
L.
Use the References to access important values if needed for this question.
Retry Entire Group 9 more group attempts remaining
grams.
L.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Application 14. Given the function f(x) = 3x3 - 48x find the following: (Round to 1 decimal place) a) The x and y intercepts [K/U: ] b) The local maximums and minimums [A:. c) Find any points of inflections [A: I d) Graph the function [C 5arrow_forwardIs there any example of liquid liquid phase separation of homogeneous mixture in our daily life?arrow_forwardwhy is a fractionating column better able to separate liquids?arrow_forward
- O lck To December- YouTub xM inbor emmareehernandes130 X e ss sc Speciic Heat S Check x+ goformative.com/formatives/6059e5322720belbcatiff322 E The Latest Gris & G a Unit 3 a Aceble Driving 0 Tarot Card Meaning 3 Tin is widely used for plating steel cans used as food containers, in metals used for bearings, and in solder. What will be the final temperature of a 163.79 g piece of tin that is at an initial temperature of 27.4°C and requires 2432.0 J of heat? The specific heat of tin is 0.226 g/J °C. Show Your Work a * 9arrow_forwardFor question look at picture regarding precision %arrow_forwardesc mb V ps lock Ft ! 1 control 141 PI Q >> A @ 2 * F2 28. NR # 80 F3 $ 888 FA % 20 FO A 244 FO & 44 F7 Aa * D'Il FB Some matches consist of a wooden stick and a head that contains tetraphosphorous trisulfide, P4S3(s), and that can be ignited on any rough surface. When the match is drawn across a rough surface, enough heat is generated to start the reaction represented by the following equation. and pur The energy that is transferred when 69.1 g of XeF4(s) is produced is DD FO Use the following information to answer the next question. Xenon tetrafluoride is a binary compound made from a noble gas. The formation of xenon tetrafluoride can be represented by the following equation. Xe(g) + 2 F₂(g) → XeF4(s) AH=-251 kJ Use the following information to answer the next two questions. DIDIOMBATILAI P4S3(s) + 8 O₂(g) → SO₂(g) A,HP,S, (S) A,HO P.O₁0(s) J F10 kJ. P4010(s) + 3 = -155.0 kJ/mol = -2 984.0 kJ/mol TULBUR di F11 F12 IN G elete 1 urn iftarrow_forward
- Pls do fast and i will rate instantly for sure Try to give solution in typed formarrow_forwardme x C Thermo X Cosmet x C Laptops x M Inbox (7 X C Buy TCL X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content T K Saturat X ZA a Amazon x C Show Ye X G what's a X Email- 1 X ☆ b The dia x h EX E ct the rato at which + 4. Consider the interconversion of A and B. Suppose in the absence of an enzyme, the forward rate constant KF is 104 s¹ and the reverse rate constant KR is 106 S¹. Calculate the equilibrium constant K. How would the presence of an enzyme affect the value of K? Karrow_forwardWhich of the following methods would be the most accurate process for delivering 20 mL of a liquid? Select one: a. using a 20 mL beaker b. using a 20 mL volumetric pipette c. using a 25 mL graduated cylinder d. using a 1 mL autopipette and measuring 1 mL twenty timesarrow_forward
- 2140.56+9.3456arrow_forwardE ! G Google Docs: Online Document Editor | Google Workspace Hiranrat_Analysis_CHM235LL DO File Edit View Insert Format Tools Extensions Help A P 100% F1 C Pure A: Pure B: Pure C: Compound 2 F2 Normal text A 146 1 BO с O junk O Crude Fractions. The following table shows a sequence of TLC "lanes" that correspond to fractions collected from a column. After completing the column, a student wants to condense containers while maintaining high purity for each of the components, then what fractions, if any, can be combined to provide # 3 Y 1 2 80 F3 O Times New... Y - 11 + 213 Oo 3 2 0 0 0 0 0 0 0 $ 4 Last edit was 2 minutes ago B IU A UA 45 S Fractions from column 000 F4 6 docs.google.com Hiranrat Analysis_CHM235LL - Google Docs % 5 F5 6 O GOD O O 2 = 2 MacBook Air F6 & 7 6 E 7 F7 Solvent Front Starting Line T 8 DII IE = E 8 9 y DD C F9 pearrow_forwardWhat characteristics of a fired bullet are individualizing? The individualizing characteristics of a fired bullet are : caliber, rifling pattern, direction of twist of the lands and grooves, number of lands and grooves. IS MY ANSWER CORRECT?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY