Concentrate on lead element. All isotopes are listed in the table. Isotope masses are listed in AMU (atomic mass unit) and value of AMU is given in Physical Constants section below this document together with other physical constants.
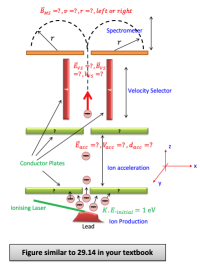
An ionizing laser is directed onto the lead block under test such that the ionized atoms (or isotopes) leaves the lead block with an initial kinetic energy of 1 eV. The direction is conically upward but sure that the cone is larger than the entrance hole to the accelerator section. The ionized isotopes are in form. That is only a single electron is missing in lead ions. The number of ionized isotopes entering into the accelerator region corresponds to a ion current.
These ions are accelerated with a constant DC voltage in the acceleration chamber which is in the form of two parallel metal plates that have a hole in both bottom and top.
Then the ions enter to velocity selector region. In this region both electric and magnetic fields are applied. Next they enter into the mass spectrometer region. In this region there is magnetic field different than the previous region. Depending of the charge of the ions, they travel in a circular path with a well-defined radius either to the left or to the right.
Physical Constants:
1 AMU=1.66×10^(-27) kg
m_neutron=1.67×10^(-27) kg
Q_e=1.60×10^(-19) Coulombs
1 eV=1.60×10^(-19) joules

Step by stepSolved in 6 steps with 7 images

- explain this phenomenon pleasearrow_forwardQuestion 1 a) The diagram below shows a plan view of the apparatus used by Rutherford and his team which led them to proposing the nuclear model of the atom. Travelling microscope with phosphorescent screen Gold foil Alpha source in lead collimator Describe how the apparatus was used to probe the microscopic structure of matter. [Suggested word count 150] b) Whilst the vast majority of alpha particles passed through the gold foil with little or no deviation, a small fraction of them were scattered through larger angles, some even backwards. Commenting on these findings Rutherford wrote “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.” Explain the implications of Rutherford’s results for models of atomic structure.arrow_forwardProblem 7: Consider what you've learned so far regarding the nucleus of an atom.arrow_forward
- Problem 1 Silicon has three naturally-occurring isotopes: 92.23% of 28Si, with an atomic weight of 27.9769 amu, 4.68% of 29Si, with an atomic weight of 28.9765 amu, and 3.09% of 30Si, with an atomic weight of 29.9738 amu. On the basis of these data, confirm that the average atomic weight of Si is 28.0854 amu.arrow_forwardPlease provide a structure consistent with the following IR, 13 C NMR, and 1 H NMR spectra. Assign at least 2 bands in the IR and assign ALL protons in the 1 H/ 13 C NMR spectrumarrow_forwardIf the radius of a calcium ion is 0.22 nm, how much energy does it take to singly ionize it? Give your answer in electron-volts (eV) with precision 0.1 eV. Give your answer to 2 significant digits.arrow_forward
- Please don't provide handwritten solution .... A sample of oxygen gas is irradiated with MgKα1α2 radiation of 0.99 nm (1253.6 eV). A strong emission of electrons with velocities of 1.57* 10^7 ms^−1 is found. What is the binding energy of these electrons?arrow_forwardQUESTION 9 In the picture are two diagrams of the first five orbits in the Bohr model labeled A - E. The dot in an orbit is the electron. The nucleus is not shown. Shown is a BEFORE and AFTER state of the atom. An energy level diagram for the Bohr hydrogen atom is shown. Which of these events has occurred in going from the BEFORE to the AFTER state? a. a photon of energy 1.51 eV has been absorbed b. an electron of energy 1.51 eV has been emitted c. a photon of energy 0.97 eV has been emitted d. a photon of energy 1.51 eV has been emitted e. a photon of energy 0.97 eV has been absorbedarrow_forwardDate 1 Page 8. Rhodium has an atomic radius of 0.345 nm and a density of 19.41 g/m³. Determine whether it has an FCC or BCC crystal structure = 102.91 g/mol) (Atomic weight of Rhodiumarrow_forward
- Label the 4 elements marked with red numbers in terms of the spherical coordinates (r,theta,phi). +y 2- 4 1 dộ dr = r sin(theta) = r sin(theta) d phi = rd phi =arrow_forwardA USATestprep, LLC - Online State t x odules/questions/qq.php?testid=1745&assignment_id3D47667397&strand=8721&element=73561&totalQuestions=.. Wages - Prote. ne to Beta PRACTICE I HELP JULIE Y GIA RESS GNMENTS ASSIGNMENT 1 23 4S 89 10 Save Submit Two identical charges are separated by a distance d. If the distance between them is increased to 3d, what will happen to the force of repulsion between them? es ) A) It wilt be one-ninth the original force. B) It will be one-third the original force. C) It will be nine times the original force. D) It will be three times the original force. Electrical and Magnetic Forces Regular Calcarrow_forwardExplain the process by which an energy dispersive X-ray spectrum (EDX) spectrum is generated and the origins of the La and Ka lines for Fe in the EDX shown in Figure 3 below. Why do the Fe La lines have a lower energy than the Ka lines? By considering the energy of the X-rays measured, discuss whether EDX can be used to measure whether the iron in an iron oxide is in 2+ or 3+ valence state? Which other spectroscopy technique in the transmission electron microscope (TEM) is more appropriate and why? OK 0.01 10.00 kev Figure 3: EDX spectrum of multi-element glass (NIST K309) containing 0, Al, Si, Ca, Ba and Fe.arrow_forward