
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
QUESTION 9
-
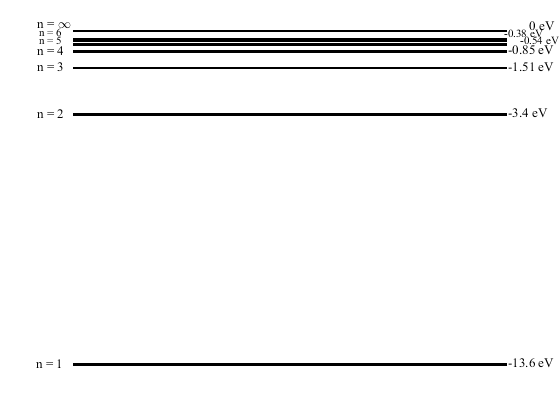
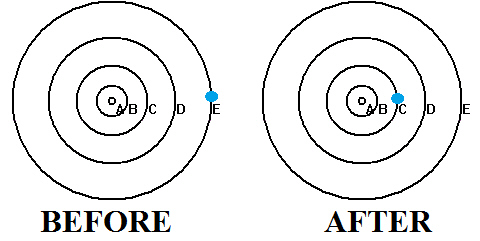
In the picture are two diagrams of the first five orbits in the
Bohr model labeled A - E. The dot in an orbit is the electron. The nucleus is not shown. Shown is a BEFORE and AFTER state of the atom. An energy level diagram for the Bohr hydrogen atom is shown. Which of these events has occurred in going from the BEFORE to the AFTER state?
a. a photon of energy 1.51 eV has been absorbed
b. an electron of energy 1.51 eV has been emitted
c. a photon of energy 0.97 eV has been emitted
d. a photon of energy 1.51 eV has been emitted
e. a photon of energy 0.97 eV has been absorbed
Transcribed Image Text:n = 3
-0.85 eV
--1.51 eV
n =2
-3.4 eV
--13.6 eV
Transcribed Image Text:B /c
JE
JE
BEFORE
AFTER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A hypothetical atom has energy levels given in the figure below. Which figure most accurately depicts the absorption spectrum, assuming all transitions between energy levels is possible? The black vertical lines represent the absorbed wavelengths. Eo = -11.5 eV E₁ = -9.6 eV E₂ = -5.2 eV O E3 = -1.1 eV wavelength increases → wavelength increases → wavelength increases → wavelength increases → wavelength increases →arrow_forwardIf the Bohr radius of the n = 3 state of a hydrogen atom is R, then the radius of the ground state is A. 9R. B. 3R. C.R/3. D. R/9. Answer is D, please explain and write clearly.arrow_forwardAn electron is excited from the n=1 ground state to the n=3 state in a hydrogen atom. Which of the following statements are true? Correct the false statements to make them true. (It may help to draw the Bohr model of the atom with the corresponding electron orbits.)a. It takes more energy to ionize (completely remove) the electron from n = 3 than from the ground state.b. The electron is farther from the nucleus on average in the n = 3 state than in the n = 1 state.c. The wavelength of light emitted if the electron drops from n = 3 to n = 2 will be shorter than the wavelength of light emitted if the electron falls from n = 3 to n = 1.d. The wavelength of light emitted when the electron returns to the ground state from n = 3 will be the same as the wavelength of light absorbed to go from n = 1 to n = 3.e. For n = 3, the electron is in the first excited state.arrow_forward
- In the picture are two diagrams of the first five orbits in the Bohr model labeled A - E. The dot in an orbit is the electron. The nucleus is not shown. Shown is a BEFORE and AFTER state of the atom. An energy level diagram for the Bohr hydrogen atom is shown. Which of these events has occurred in going from the BEFORE to the AFTER state? a. a photon of energy 0.97 eV has been emitted b. a photon of energy 1.51 eV has been absorbed c. a photon of energy 1.51 eV has been emitted d. an electron of energy 1.51 eV has been emitted e. a photon of energy 0.97 eV has been absorbedarrow_forwardAn electron is orbiting in the n = 3 orbit of an hydrogen atom. It is promoted by absorption of light energy to the n = 4 level. The Rydberg constant is R = 1.097 x 107 m-1. What is the wavelength of the light absorbed? Select one: a. 427 nm b. 1.094 μm c. 1.875 μm d. 763 nmarrow_forwardThe Balmer series in hydrogen includes the Paschen series. has four lines in the ultraviolet. O includes both the Paschen series and the Lyman series. O has four lines in the visible. O includes the Lyman series.arrow_forward
- In the ground state of the Hydrogen atom the energy of the electron is E0 = -13.61 eV. What is the energy of the electron in the ground state of the He+ ion? Hints:The He+ ion is a Hydrogen-like structure, it has only one electron.How does the energy of the electron depend on the charge of the nucleus? Is this a bound state? Make sure, your answer has the correct sign. Incorrect. Tries 1/20 Previous Tries What is the energy of the electron in the ground state of the Li++ ion? Tries 0/20 The electron in the He+ ion is excited to the n = 2 principal state. What is the energy of the electron now? Tries 0/20 What is the energy of the electron in the Li++ ion in the n = 2 principal state? Tries 0/20 What is the energy of the electron in the Li++ ion in the n = 3 principal state? Tries 0/20 Take element Z = 83 from the periodic table. Ionize it 82 times so that there is only one electron left orbiting around the nucleus. What is the…arrow_forwardAn electron is orbiting in the n = 3 orbit of an hydrogen atom. It is promoted by absorption of light energy to the n = 4 level. The Rydberg constant is R = 1.097 x 107 m-1. What is the wavelength of the light absorbed? Select one: a. 763 nm b. 427 nm c. 1.094 μm d. 1.875 μm Clear my choice ◀︎ Workshop week 11 2020 Fission and Fusion Solutionsarrow_forwardQuestion in imagesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON