
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ewtty, Carlos
3/33 answered O
Show
Remaining
Please save all answers. Questions that you save can still be ec
nave 1 UNSAVED ANSWER -
SAVE ALL ANSWERS
50
102
100
120
200
135
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select
an answer.
a
800
b
100
1200
d
2000
e
90
f
10
30
180
360
![Time
3/33 answered Ở
Show
Hewtty, Carlos
Remaining
Please save all answers. Questions that you save can still be edi
pu have 1 UNSAVED ANSWER-
SAVE ALL ANSWERS
Question 32
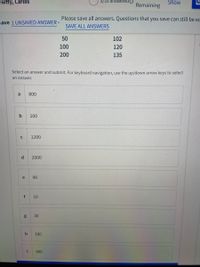
The initial rate for an enzyme-catalyzed reaction has been determined at
several substrate concentrations. Data are as follows:
The Km value (µM) is:
TT
[S] umol/L
v[(umol/L) min-1]
22
10
39
20
65
50
102
100
120
200
135
Select an answer and submit. For keyboard navigation, use the up/down arrow kéys to select
an answer.
a
800
100
1200
2000
e
90
10](https://content.bartleby.com/qna-images/question/d5cc39c9-8b2b-4beb-a3cb-5d1bdcb587ab/705851e9-c56d-4b44-9939-1e8853d285b6/erxsf_thumbnail.jpeg)
Transcribed Image Text:Time
3/33 answered Ở
Show
Hewtty, Carlos
Remaining
Please save all answers. Questions that you save can still be edi
pu have 1 UNSAVED ANSWER-
SAVE ALL ANSWERS
Question 32
The initial rate for an enzyme-catalyzed reaction has been determined at
several substrate concentrations. Data are as follows:
The Km value (µM) is:
TT
[S] umol/L
v[(umol/L) min-1]
22
10
39
20
65
50
102
100
120
200
135
Select an answer and submit. For keyboard navigation, use the up/down arrow kéys to select
an answer.
a
800
100
1200
2000
e
90
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- 49 A 1 litre infusion of sodium chloride 0.9% is being given at a rate of 28 drops/min (standard giving set). How long will the infusion run at the specified rate?arrow_forwardInterpret the following ABG results: PH 7.48, PAC02 50 mm Hg, HCO3- 32 mEq/L: Interpret the following ABG results: PH 7.23, PAC02 40 mm Hg, HCO3- 19 mEq/L: Interpret the following ABG results: PH 7.48, PAC02 18 mm Hg, HCO3- 13 mEq/L:arrow_forwardWhat term describes kcat/Km? What is the maximum value that this term can attain? Explain.arrow_forward
- Siturul alORK ING )A mother brings her 251b child Into the ER with q very high fevero she has already ad ministered l2 4sp of Tylenol at home. She has brought the bottle with her and the label states: Acetamaino phere syrmp 325 mg / 5mLo Administer 10-15 mg /89 every 4 hrs as needed . How many milligrams oL Tylenot has she givern? Was this asafe dose to give the child?arrow_forward12. 108 cm = inarrow_forwardCalculate the dose in Mg: What is the dose of a patient weighing 30 kg, if the dose is 10 mg/kg?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON