
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
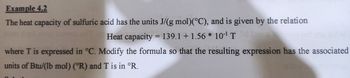
Transcribed Image Text:Example 4.2
The heat capacity of sulfuric acid has the units J/(g mol)(°C), and is given by the relation
Heat capacity = 139.1 +1.56* 10¹¹ T
where T is expressed in °C. Modify the formula so that the resulting expression has the associated
units of Btu/(lb mol) (R) and T is in °R.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- What amount of heat energy is required to heat 2.50 grams of water at 45°C (c = 4.18 J/g°C) to 85°C? er]arrow_forwardNumber 1A food product containing 82% moisture content is being frozen. Estimate the specific heat of the product at -8°C when 82% of the water is frozen. The specific heat of the dry product is 2,5 kJ/(kg°C). It is assumed that the specific heat of water at -10°C is the same as the specific heat of water at 0°C, and that the specific heat of ice follows the function Cp es = 0.0062 Tbeku + 2.0649. identify :a. Cp frozen product = Answer in kJ/kg°Carrow_forwardQ.3 Two formulas for the heat capacity of CO are given here: C,(cal/(mol-"C)] = 6.890 + 0.0014367(°C) C,(Btu/(lb-mole "F)] = 6.864 + 0.00079787('F) Starting with the first formula, derive the second. (Recall Section 2.5, and remember that the tem- perature unit in the denominator of C, refers to a temperature interval.)arrow_forward
- Using the thermochemical equation below, what volume of chlorine gas is produced given that 320.00 kJ are also produced? _02 + _HCI → _Cl2 + _H2O ΔΗ - -202.60 kJarrow_forwardJ 5arrow_forwardVapor pressure data are given here for octane, C8H18. Temperature(c) Vapor Pressure (mm Hg) 25 13.6 50 45.3 75 127.2 100 310.8 Use the Clausius–Clapeyron equation to calculate the molar enthalpy of vaporization of octane and its normal boiling pointarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The