
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
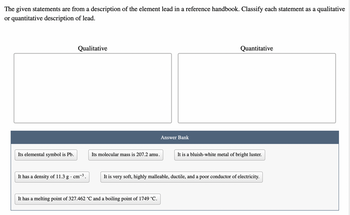
Transcribed Image Text:The given statements are from a description of the element lead in a reference handbook. Classify each statement as a qualitative
or quantitative description of lead.
Its elemental symbol is Pb.
Qualitative
It has a density of 11.3 g . cm-³.
Its molecular mass is 207.2 amu.
Answer Bank
It has a melting point of 327.462 °C and a boiling point of 1749 °C.
Quantitative
It is a bluish-white metal of bright luster.
It is very soft, highly malleable, ductile, and a poor conductor of electricity.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using any data you can find in the ALEKS Data resource, calculate the equilibrium constant K at 25.0 °C for the following reaction. + 4 HCl (g) TiCl (g) + 2 H₂O(g) ― TiO2(s) Round your answer to 2 significant digits. K = ☐ ☐ ☑arrow_forwardA student finds that they have 0.2545 g of NH4Cl, 1.1502 g of NaCl, and 1.0181 g of SiO2. Calculate the percent gained or lost during this experiment if the initial mass of the mixture was 2.5537 g.arrow_forwardA chemistry student must write down in her lab notebook the concentration of a solution of potassium chloride. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 1.55 g • She put some solid potassium chloride into the graduated cylinder and weighed it. With the potassium chloride added, the cylinder weighed 31.436 g. • She added water to the graduated cylinder and dissolved the potassium chloride completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 166.4 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits. -1 g.mL x10 × Śarrow_forward
- Based on this. What was the percentage by mass of water in the sample?arrow_forwardCrime scene investigators keep a wide variety of compounds on hand to help with identifying unknown substances they find in the course of their duties. One such investigator, while reorganizing their shelves, has mixed up several small vials and is unsure about the identity of a certain powder. Elemental analysis of the compound reveals that it is 67.31 % carbon, 6.978% hydrogen, 4.617% nitrogen, and 21.10% oxygen by mass. Which of the compounds could the powder be? C17H19NO3C17H19NO3 = morphine, analgesic C17H21NO4C17H21NO4 = cocaine, illicit drug C7H5N3O6C7H5N3O6 = 2,4,6-trinitrotoluene (TNT), commonly used explosive C10H15NC10H15N = methamphetamine, stimulant C4H5N2OC4H5N2O = caffeine, stimulant C11H15NO2C11H15NO2 = 3,4-methylenedioxymethamphetamine (MDMA), illicit drug C21H23NO5C21H23NO5 = heroin, illicit drug C3H6NO3C3H6NO3 = hexamethylene triperoxide diamine (HMTD), commonly used explosivearrow_forwardPlease answer number 7arrow_forward
- 2. A student was asked to identify a sample of unknown hydrate. Student was provided with the following experimental data. 3.51 g sample of the hydrate when heated loses water completely and the mass of the residue was found to be 2.22 g. Student was provided with two reference compounds from which student needs to identify the unknown: Cu(NO3)2. 3H2O and Cu(NO3)2. 6H20. Answer the followings. a. Calculate percent water of hydration in the sample. (show your calculations)arrow_forwardWhat is the volume (in mL) of 0.684 mol of carbon disulfide (MM = 76.13 g/mol) at 20 degrees celsius? The density of carbon disulfide is 1.263 g/mL at 20 degrees celsius. Your answer should have 3 sig figs.arrow_forwardYou are given a 15.0 g mixture. You find out that the mixture contains 3.55 g salt, 4.78 g sand and the rest is Iron. According to these data, what is the percent of iron in the mixture? O 55.5% O 6.67% O 44.5% 15,0%arrow_forward
- Assuming that the copper and lead are pure, determine the relative amounts of each kind of BB. The density of copper is 8.96 g/cm ^ 3 . The density of lead is 11.4 g/cm ^ 3arrow_forwardPart A: How many atoms of hydrogen does it contain? Express your answer using four significant figures. Part B: How many molecules of glucose does it contain? Express your answer using four significant figures. Part C: How many moles of glucose does it contain? Express your answer using four significant figures. Part D: What is the mass of this sample in grams? Express your answer using four significant figures.arrow_forwardWhat is the concentration, in units of grams of dye per liter of solution (g/L), of the dilute dye solution that is produced as follows? Transfer 16.51 mL of the stock solution (6.12 g/L) to a 200.0 mL volumetric flask and dilute to the 200.0 mL line with deionized water. Your answer should be reported to the correct number of significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY