
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I don’t know how to do this question
Transcribed Image Text:ssignment/takeCovalentActivity.do?locator=assignment-take
国R
B Homepage - Geor. Editor - Citation M...
Home | Navigate m HBO Max
MyLab & Masterin. * Cengage Learning
se:.
[References)
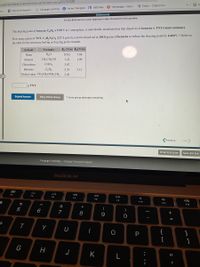
Use the References to access important values if needed for this question.
The freezing point of benzene C,H, is 5.50°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in benzene is TNT (trinitrotoluene).
How many grams of TNT, C,H<N30, (227.1 g/mol), must be dissolved in 269.0 grams of benzene to reduce the freezing point by 0.400°C ? Refer to
the table for the necessary boiling or freezing point constant.
Solvent
Formula
K, (°C/m) Kr (°C/m)
Water
H2O
0.512
1.86
Ethanol
CH;CH,OH
1.22
1.99
Chloroform
CHCI3
3.67
Benzene
CH6
2.53
5.12
Diethyl ether CH3CH,0CH,CH3
2.02
g TNT.
Submit Answer
Retry Entire Group
7 more group attempts remaining
Previous
Next
Email Instructor
Save and Exit
Cengage Learning | Cengage Technical Support
MacBook Air
F4
DII
DD
F7
F8
F9
F10
F11
F12
&
5
6
7
)
8
9
+
T
Y
{
}
[
G
H
J
K
L
+ ||
....
Expert Solution
arrow_forward
Step 1
Given:
Depression in freezing point =
Molar mass of TNT = 227.1 g/mol
Mass of benzene = 269.0 g
Kf of benzene =
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 24.34 Draw the product of each of the following reactions. piease show Allarrtw pasheng wedranonsarrow_forwardCH3 1) Hg(OAc)2 H20 a) 2) NaBH4 H3C CH3 1) BH3 D 2) H,OH,0arrow_forwardPlease answer questions A through C I could not read the handwriting last time so please make sure I can read it please. PLEASE make sure to answer all questions (questions A through C)arrow_forward
- question help pleasearrow_forwardCONCLUSIONS: In general, the boiling points of compounds increase down a group in the periodic table. The melting points and boiling points for the hydrogen compounds of group 6A elements are in the table below. Melting Point (°C) Boiling Point (°C) H₂O 0.0 100.0 H₂S -82.0 -60.0 H₂Se -65.7 -41.2 H₂Te -49.0 -2.2 Use your understanding of chemistry to propose an explanation for the anomaly in the trend for the hydrogen compounds of group 6A elements shown in the table.arrow_forward-NH2 но NH b. OHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY