
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
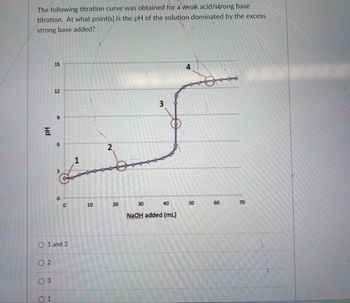
Transcribed Image Text:The following titration curve was obtained for a weak acid/strong base
titration. At what point(s) is the pH of the solution dominated by the excess
strong base added?
15
12
Hd
Hj
6
2
о
10
O 1 and 3
02
03
1
220
20
3
4
30
40
50
NaOH added (mL)
60
60
220
70
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Sketch a titration curve for the titration of potassium hydroxide with HCl, both 0.100 M. Identify three regions in which a particular chemical species or system dominates the acid-base equilibria.arrow_forwardWhen 40.00 mL of a weak monoprotic acid solution is titrated with 0.100-M NaOH, the equivalence point is reached when 35.00 mL base has been added. After 20.00 mL NaOH solution has been added, the titration mixture has a pH of 5.75. Calculate the ionization constant of the acid.arrow_forwardThe titration of 0.100 M acetic acid with 0.100 M NaOH is described in the text. What is the pH of the solution when 35.0 mL of the base has been added to 100.0 mL of 0.100 M acetic acid?arrow_forward
- Sketch the titration curve for a weak acid titrated by a strong base. When performing calculations concerning weak acidstrong base titrations, the general two-slep procedure is to solve a stoichiometry problem first, then to solve an equilibrium problem to determine the pH. What reaction takes place in the stoichiometry part of the problem? What is assumed about this reaction? At the various points in your titration curve, list the major species present after the strong base (NaOH, for example) reacts to completion with the weak acid, HA. What equilibrium problem would you solve at the various points in your titration curve to calculate the pH? Why is pH 7.0 at the equivalence point of a weak acid-strong base titration? Does the pH at the halfway point to equivalence have to be less than 7.0? What does the pH at the halfway point equal? Compare and contrast the titration curves for a strong acidstrong base titration and a weak acidstrong base titration.arrow_forwardDetermine the dominant acid-base equilibrium that results when each of the following pairs of solutions is mixed. Indicate the equilibrium by writing 1 for a strong acid, 3 for a weak acid, 4 for an acidic buffer, 7 for a neutral solution, 10 for a basic buffer, 11 for a weak base, and 13 for a strong base. (a) 10.0 mL of 0.15 M NaOH + 15.0 mL of 0.10 M HNO3 (b) 25.0 mL of 0.10 M HCl + 10.0 mL of 0.25 M NH3 (c) 50.0 mL of 0.050 M NaOH + 50.0 mL of 0.10 M NH3 (d) 50.0 mL of 0.10 M NH3 + 50.0 mL of 0.05 M HClarrow_forwardWhat is an acidbase indicator? Define the equivalence (stoichiometric) point and the end point of a titration. Why should you choose an indicator so that the two points coincide? Do the pH values of the two points have to be within 0.01 pH unit of each other? Explain.arrow_forward
- A solution of weak base is titrated to the equivalence point with a strong acid. Which one of the following statements is most likely to be correct? a The pH of the solution at the equivalence point is 7.0. b The pH of the solution is greater than 13.0. c The pH of the solution is less than 2.0. d The pH of the solution is between 2.0 and 7.0. e The pH of the solution is between 7.0 and 13.0. The reason that best supports my choosing the answer above is a Whenever a solution is titrated with a strong acid, the solution will be very acidic. b Because the solution contains a weak base and the acid (titrant) is used up at the equivalence point, the solution will be basic. c Because the solution contains the conjugate acid of the weak base at the equivalence point, the solution will be acidic.arrow_forwardA 25.0-mL sample of hydroxylamine is titrated to the equivalence point with 35.8 mL of 0.150 M HCl. a What was the concentration of the original hydroxylamine solution? b What is the pH at the equivalence point? c Which indicators, bromphenol blue, methyl red, or phenolphthalein, should be used to detect the end point of the titration? Why?arrow_forwardGiven three acid-base indicators—methyl orange (end point at pH 4), bromthymol blue (end point at pH 7), and phenolphthalein (end point at pH 9)—which would you select for the following acid-base titrations? (a) perchloric acid with an aqueous solution of ammonia (b) nitrous acid with lithium hydroxide (c) hydrobromic acid with strontium hydroxide (d) sodium fluoride with nitric acidarrow_forward
- A student intends to titrate a solution of a weak monoprotic acid with a sodium hydroxide solution but reverses the two solutions and places the weak acid solution in the buret. After 23.75 mL of the weak acid solution has been added to 50.0 mL of the 0.100 M NaOH solution, the pH of the resulting solution is 10.50. Calculate the original concentration of the solution of weak acid.arrow_forwardKa for formic acid is 1.7 104 at 25C. A buffer is made by mixing 529 mL of 0.465 M formic acid, HCHO2, and 494 mL of 0.524 M sodium formate, NaCHO2. Calculate the pH of this solution at 25C after 110 mL of 0.152 M HCl has been added to this buffer.arrow_forwardMalonic acid (HO2CCH2CO2H) is a diprotic acid. In the titration of malonic acid w ith NaOH, stoichiometric points occur at pH = 3.9 and 8.8. A 25.00-mL sample of malonic acid of unknown concentration is titrated with 0.0984 M NaOH, requiring 31.50 mL of the NaOH solution to reach the phenolphthalein end point. Calculate the concentration of the initial malonic acid solution. (Sec Exercise 113.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning