
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
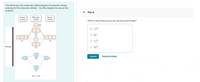
Transcribed Image Text:The following is the molecular orbital diagram showing the energy
ordering for the molecular orbitals. Use this diagram to answer the
question.
Part A
Molecular
orbitals
Atomic
orbitals
Atomic
orbitals
Which of the following has the shortest bond length?
O C,2+
O B*
2p
2p
O c,2-
Energy
O N,2+
Submit
Request Answer
B2, C2, N2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 15arrow_forwardIs This correctarrow_forwardUse Lewis structures, molecular shape assignments, electronegativities, and symmetry to determine whether each of the following has (a) a net dipole and (b) would be classified as polar or non-polar. A) BF3 b). HCN c). F2O. d) SF6 E) CS2 f) NNOarrow_forward
- Select the items below that are properties of sigma bonds options: sidewise overlap of atomic orbitals stronger bonds head-on overlap of atomic orbitals double or triple covalent bonds weaker bonds single covalent bond Select the items below that describe pi bonds options: single covalent bond stronger bonds sidewise overlap of atomic orbitals weaker bonds head on overlap of atomic orbitals double or triple covalent bondarrow_forwardYou will need to draw the correct Lewis structure for BrH4 to answer the following questions. A) How many nonbonded electron pairs are on the Br? B) How many bonded electron pairs are in Br valance shell? C) Name the molecular geometry? D) What is the H-Br-H bond angle? E) What is hybridization on the Br atom?arrow_forward... Question 10 of 18 A TT bond could be formed from the overlap of which two orbitals? A) two sp² hybrid orbitals B) a 1s and a sp² hybrid orbital C) a sp and a sp² hybrid orbitals D D) two unhybridized p orbitals Stax Periodic Table 68% Submitarrow_forward
- What molecules have trigonal planar ELECTRON PAIR GEOMETRY A) O3 B) HCl C) ClO3- D)NaCl E)CH2O F) BF3 G) BrF3arrow_forwardO CHEMICAL BONDING Recognizing typical LCAO molecular orbitals Heather V Stua, che following sketch of a molecular orbital (MO) in a homonuclear diatomic molecule. This MO was formed by combining one 3p atomic orbital from each atom. The dark dots in this sketch are the nuclei. do Now use the sketch to complete the table below. Write the symbol for this MO. O bonding O antibonding O higher Is this a bonding or antibonding MO? On O lower What is the energy of this MO, compared to the energy of a 3p orbital on one of the separate atoms? O the same O not enough information to decide Explanation Check O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy| Accessibilityarrow_forwardWhich of the following molecular formulas, once the BEST lewis structure has benn solved always lead to a molecule which is non polar because it possesses no net dipole moment? a) SF4 b) H2O c) CH2O d) NH3 e) BF3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY