
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
The following image is a schematic of the
A=Light Dependent reactions
B=Glycolysis
C=Light Independent Reactions
D=Kreb's cycle
E=electron transport chain
2a) Use the figure above to explain the conditions in which the oxygen concentration remains constant in the container.
b) 2-carboxyarabinitol-1,5-bisphosphate (CABP) is an inhibitor of the enzyme rubisco. Which pathway would this affect most?
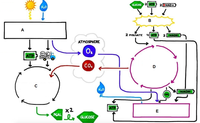
Transcribed Image Text:ATP
2INAD+
GLUCOSE
H2O
В
A
2 PYRUVATE
4 ATP
2 NADH
ATMOSPHERE
ATP
NADPH
CO.
D
H20
NADH
ATP
x2
PGAL
l GLUCOSE
E
be
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- I have an assignment about aerobic respiration, anaerobic respiration, and fermentation in e coli. I am supposed to compare and contrast the methods, then list, where applicable, terminal electron acceptors, oxidized and reduced products, fermentation products, modes of ATP synthesis, and relative amounts of ATP produced for each. I've been working on this question all week and now I'm just lost. Wouldn't all three start at glycolysis? And in aerobic respiration, I know that glucose gets reduced to pyruvate in glycolysis, and that goes for all bacteria, right? Thank you for any help you can give me.arrow_forwardOne of the examples that we have used to illustrate the concept of equilibrium is the isomerization of glucose-6-phosphate (G6P) to fructose-6-phosphate (F6P), which is the second step in g ycolysis. Draw a graph to show how the reaction Gibbs energy varies with the fraction fof F6P in solution.Label the regions of the graph that correspond to the formation of F6P and G6P being spontaneous, respectively.arrow_forwarda) What happens during the two phases of glycolysis? Write the reaction steps of glycolysis showing their the enzymes that catalyze the reactions. (Draw only the structure of the first and last product) b) How many net moles of ATP can be synthesized from each mol of glucose? c) The overall equation for glycolysis?arrow_forward
- Remember that hexose kinase is an enzyme required for hexose metabolism, a process that takes a 6-carbon sugar and breaks it down into 2 pyruvate molecules. Practice Question 5 A) What is a specific metabolic process in which hexose metabolism occurs?arrow_forwardOne of the steps in psilocybin biosynthesis is the phosphorylation of 4-hydroxytryptamine By the enzyme Psik as shown below, that involves the hydrolysis of ATP. NH₂ OH HO Psik HO of A& ADP 4-hydroxytryptamine ATP norbaeocystin The standard free energy changes for each reaction is shown in the Table below. J reaction 4-hydroxytryptamine + P₁ → norbaeocystin ATP → ADP+ Pi (b) What is the equilibrium constant at 37 °C? AG 27.7 kJ/mol -32.2 kJ/mol (a) Write the net reaction. What is the standard free energy change for the net reaction? (c) In the cell, the concentration of ATP is 3.1 mM, the concentration of P; Is 5.90 mM, and the Concentration of ADP is 220 μM. What will AG Be if the concentration of norbaeocystin Is always kept at 1/100 of the concentration of 4-hydroxytryptamine?arrow_forwardWhich of the following describes a purpose for the function of phosphoglucoisomerase? a) allows for phosphorylation for trapping the molecule in the cell b)promotes substrate level phosphorylation and formation of ATP c) allows formation of a primary alcohol necessary for the next step of phosphorylation d)facilitates epimerization, the change in stereochemistry at one chiral carbonarrow_forward
- Intermediates of a pathway are shown in the following scheme. Using curved arrows, show the mechanism of each step labeled with a blue letter. Draw out abbreviated structures of the coenzymes, so that you can effectively show all arrow pushing. You may abbreviate the coenzymes by putting R groups on the molecule, but do draw out the parts of the structure that are involved in the arrow pushing. Some of the transformations will require you to show multiple structures to show all of the arrow pushing (particularly some of the coenzyme-mediated steps). You do not need to show specific amino acid residues that perform the catalysis. You can abbreviate acidic amino acid residues “Enz–B–H” and basic residues “B–Enz”.arrow_forwardConcerning the use of pyruvate when ratio of NADH/NAD+ is low, what is the fate of the carbon labeled in pyruvate when metabolized under these conditions? a) Production of glucose b) Oxidation to CO2 via the TCA cycle c) Conversion to pyruvate to generate oxaloacetate to move electrons to the cytosol via the malate shuttle d) Pyruvate is never metabolized by the cell, instead pyruvate is converted to lactate and solely exported for the Cori cycle and dependent on the liver to recycle the carbon skeletonarrow_forwardThe major control enzyme in glycolysis is: a) hexokinase b) phosphofructokinase c) aldolase d) pyruvate kinase In step 4, when fructose-1,6-biphosphate is cleaved, the two molecules formed are: a) GAP and DHAP b) GAP and 3-PG c) 3-PG and 2-PG d) PEP and pyruvate NAD is used in which step? A) conversion of DHAP to GAP b) conversion of GAP to 1,3-BPG c) conversion of 1,3-BPG to 3-PG d) conversion of PEP to pyrunate The number of ATP molecules formed at the substrate level in glycolysis is: a) 1 B) 2 c) 3 d) 5 The number of ATP molecules formed indirectly via oxidative phosphorylation in glycolysis is: a) 1 B) 2 c) 3 d) 5arrow_forward
- Which of the following do cellular respiration and fermentation have in common? There might be more than one answer.  A) Both of these pathways provide a mechanism for oxidation of glucose B) The citric acid cycle is involved in both of these pathways C) Enzymes are involved in both of these pathways D)ATP synthase is required to complete both of these pathways E)Both of these pathways occur within mitochondria F) ATP is generated in both of these pathways .arrow_forwardUse the graph below to determine which of the following is the optimal temperature for catalase. Catalase Activity 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 10 20 30 40 50 60 Temperature (°C) O 0.8 O 1.45 O 37 O 20 Percent Increase in Oxygen per Minutearrow_forwardWe eat foods containing sucrose (table sugar), lactose (milk sugar), and cellobiose (disaccharide of cellulose in plants). How many net moles of ATP per mole disaccharide do humans generate from the disaccharides sucrose, lactose, and cellobiose using the glycolytic pathway? Osucrose: 4 ATP; lactose: 2 ATP; cellobiose: 2 ATP Osucrose: 2 ATP; lactose: 4 ATP; cellobiose: 2 ATP Osucrose: 2 ATP; lactose: 2 ATP; cellobiose: 0 ATP sucrose: 6 ATP; lactose: 2 ATP; cellobiose: 0 ATP sucrose: 4 ATP; lactose: 4 ATP; cellobiose: 0 ATParrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON