The following data were measured in a 1.00-cm cell. Concentration (M) %T 14.9 26.1 38.0 54.3 65.7 1.50x104 1.00x104 7.50x10-5 5.00x10-5 3.00x10-5 Determine the molar absorptivity( in cm ¹M1) using graphical methods.
The following data were measured in a 1.00-cm cell. Concentration (M) %T 14.9 26.1 38.0 54.3 65.7 1.50x104 1.00x104 7.50x10-5 5.00x10-5 3.00x10-5 Determine the molar absorptivity( in cm ¹M1) using graphical methods.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.23QAP: The following cell was found to have a potential of —0.492 V: Ag|AgCl(sat’d)||HA(0.200 M),NaA(0.300...
Related questions
Question
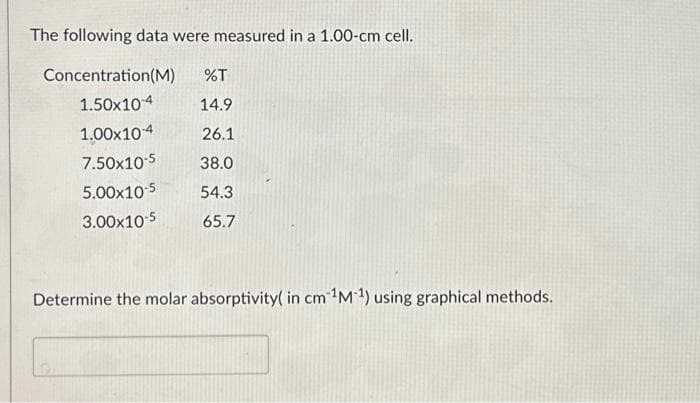
Transcribed Image Text:The following data were measured in a 1.00-cm cell.
Concentration (M) %T
1.50x10-4
14.9
1.00×10-4
26.1
7.50x10-5
38.0
5.00x10-5 54.3
3.00x10-5
65.7
Determine the molar absorptivity( in cm ¹M-¹) using graphical methods.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 13 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning