
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
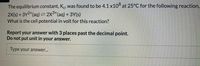
Transcribed Image Text:The equilibrium constant, Kc, was found to be 4.1 x108 at 25°C for the following reaction,
3+
2X(s) + 3Y2+ (aq) = 2X³+ (aq) + 3Y(s)
What is the cell potential in volt for this reaction?
Report your answer with 3 places past the decimal point.
Do not put unit in your answer.
Type your answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the cell potential (Ecell) at 25oC (298 K) for the following reaction if the Cu2+ ion concentration is 0.061 M and the Fe2+ ion concentration is 0.84 M. Report your answer with 3 decimal places. Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s) Half-reaction Standard Reduction Potential (V) Fe2+(aq) + 2e−→ Fe(s) −0.440 Cu2+(aq) + 2e−→ Cu(s) +0.337 R = 8.31 V/mol·K F = 96500 C/molarrow_forwardZn In a zinc-copper cell, |Zn 2 (aq) || Cu+2 (aq) |), which electrode is positive? A Cu 2 CU) C Zn 2arrow_forwardFor a standard voltaic cell comprised of a Cu2+|Cu electrode and a Fe2+|Fe electrode, what is the cell potential, in volts?arrow_forward
- What is E at 25 °C for the reaction described by Mg(s) | Mg²⁺ (1.60 M) || Pb²⁺ (0.850 M) | Pb(s)? The standard cell potential for the reaction is +2.231 V.arrow_forwardThe standard potential for the reaction below is -1.036 V at 25°C. 3 Y+ (aq) + X (s) → 3 Y (s) + X+3 (aq) Calculate the value of the equilibrium constant for the reaction at 25°C. [Do not be surprised by very large or very small values. Large or small values should be entered in scientific notation.] Example: 3.40×10-6 = 3.40E-6 Example: 8.22×1020 = 8.22E20 K = Answerarrow_forwardUse the tabulated electrode potentials to calculate △Grxn for the following reaction at 25 °C2 Fe^3 (aq) + 3Sn(s) ---> 2Fe (s) + 3Sn^2 (aq)arrow_forward
- Consider the reaction corresponding to a voltaic cell and its standard cell potential. Zn(s)+Cu2+(aq)⟶Cu(s) +Zn2+(aq) Eocell=1.1032 V What is the cell potential for a cell with a 2.214 M solution of Zn2+(aq) and 0.1456 M solution of Cu2+(aq) at 437.7 K?arrow_forwardCalculate the cell potential of each of the following electrochemical cell at 25oC. a. Pt|H 2 (8.00 atm)|H + (1.00 x 10 -3 M)‖Ag + (0.00549 M)|Agb. Pt|H 2 (1.00 atm)| H + (pH = 5.97 ) ‖ H + (pH=3.47)|H 2 (1.00 atm)|Ptc. Pt|H 2 (0.0361 atm)|H + (0.0175 M)‖H + (0.0175 M)|H 2 (5.98 x 10 -4 atm)|Pt Please solve and give explanations steps.arrow_forwardDetermine the cell potential (in volts) of a standard galvanic cell constructed using iron and lead metals and their associated solutions, 1M FeSO4 and 1M PbSO4.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY