
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
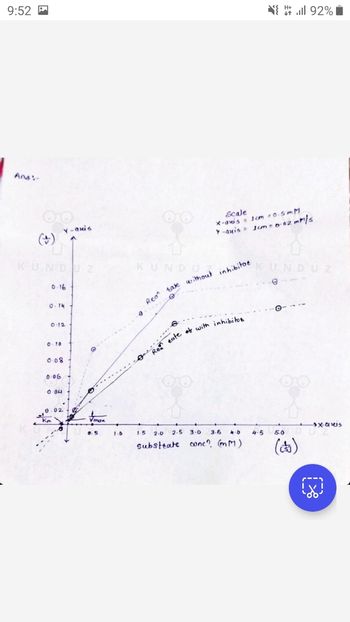
Can u do only calculation?? And check my graph if correct just Re write my graph and do calculation okk
Transcribed Image Text:9:52
92%
Ans:-
(4)
Y-axis
DUZ
KU
0.16
3.14
KUNDU
Scale
X-axis 1cm 0.5mM
Y-axis = 1cm = 0.62 mm/s
Rean take without inhibito
KUNDUZ
0.12
0.10
0·08
Re tate of with inhibitor
0.06
0 04
0.02
Кт
max
яхах
0.5
1.6
1.5
2.0 2.5 3.0
3.5 4.6
4.5
5.0
substrate conc? (mm)
(do)
☑
![Directions: Solve the following problem:
The enzyme ẞ-methylaspartase catalyzes the deamination of ẞ-methylaspartate:
CH,NH,
CH₁₂
OOC-CH-CH-COOOOC-CH-CH-COO+NH
mesaconate
[V. Williams and J. Selbin, J. Biol. Chem. 239, 1636 (1964)]
The effects of hydroxymethylaspartate as an inhibitor for this enzyme was studied. The following data wer
Substrate Concentration
obtained:
Reaction Rate without
inhibitor
(mM)
1 x 10-4
5 × 10-4
1.5 x 10-3
2.5 x
10-3
5 x 10-3
(mM/s)
0.026
0.092
0.136
0.150
0.165
Reaction Rate with inhibitor
(mM/s)
0.010
0.040
0.086
0.120
0.142
Use Lineweaver-Burk plot to determine the KM and Vmax of the enzyme in the absence of inhibitor.
Moreover, determine as well whether the inhibitor is competitive or noncompetitive. Show the graphs and
calculations below.](https://content.bartleby.com/qna-images/question/d3cf5230-7915-4462-bd07-8b78c6b9bdec/e8f7d5cf-21f9-4c11-a971-ba3ba15c8c1e/bqi0qep_thumbnail.jpeg)
Transcribed Image Text:Directions: Solve the following problem:
The enzyme ẞ-methylaspartase catalyzes the deamination of ẞ-methylaspartate:
CH,NH,
CH₁₂
OOC-CH-CH-COOOOC-CH-CH-COO+NH
mesaconate
[V. Williams and J. Selbin, J. Biol. Chem. 239, 1636 (1964)]
The effects of hydroxymethylaspartate as an inhibitor for this enzyme was studied. The following data wer
Substrate Concentration
obtained:
Reaction Rate without
inhibitor
(mM)
1 x 10-4
5 × 10-4
1.5 x 10-3
2.5 x
10-3
5 x 10-3
(mM/s)
0.026
0.092
0.136
0.150
0.165
Reaction Rate with inhibitor
(mM/s)
0.010
0.040
0.086
0.120
0.142
Use Lineweaver-Burk plot to determine the KM and Vmax of the enzyme in the absence of inhibitor.
Moreover, determine as well whether the inhibitor is competitive or noncompetitive. Show the graphs and
calculations below.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Similar questions
- C Clever | Portal 04.core.learn.edgenuity.com/Player/ chools X marks -ster 2 SC211CR Credit Recovery 2019 Assignment XX MPS Biology Semester 2 SC211 X Answer questions based on the lab activity. Throughout the reflection, make sure you have a copy of the Student Guide and your data tables. Fill in the terms that complete the statements. + Intro In this lab, you observed how changes in the environment can affect the health of a watershed. You saw how two activity affected types of entered and flowed through the watershed. You also predicted how [ food chains, diagrams that show how energy is passed from one biotic factor to another through the foods they eat. ☐☐☐☐☐☐☐ 1 of 12 acer M ✔Done Englisharrow_forwardGenetics Practice Problems 1. For each genotype below, indicate whether it is heterozygous (He) or homozygous (Ho) AA Bb Ee li Mm f Jj nn Ce GG kk 00 Dd HH LI Pp 2. For each of the genotypes below determine what phenotypes would be possible. Purple flowers are dominant to white Brown eyes are dominant to blue PP BB Pp Bb PP bb Round seeds are dominant to wrinkled Bobtails are recessive (to long tails) RR TT Rr Tt IT tt 3. For each phenoty pe below, list the genotypes (remember to use the letter of the dominant trait) Straight hair is dominant to curly straight Tail spikes are dominant to plain tails spikes straight spikes curly plain 4. Set up the Punnet squares for each of the crosses listed below. Round seeds are dominant to wrinkled. Rr x rr What percentage of the offspring will be round? Rr x Rr What percentage of the offspring will be round? RR x Rr What percentage of the offspring will be round? | ||arrow_forwardCan you please answer 31arrow_forward
- please help me with the following question asap please its important!! State your null hypothesis for the flower experiment, giving the probability of a left-handed flower # and probability of a right-handed flower (assuming right-handed is dominant) and what is the alternative hypothesis? Null hypothesis : Alternative hypothesis:arrow_forwardI need help with this Biology Homework Question.arrow_forwardNEED A HELP WITH THIS QUESTION FOR BIOarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON