
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
How can we solve this reaction?
Transcribed Image Text:Macmillan Learning
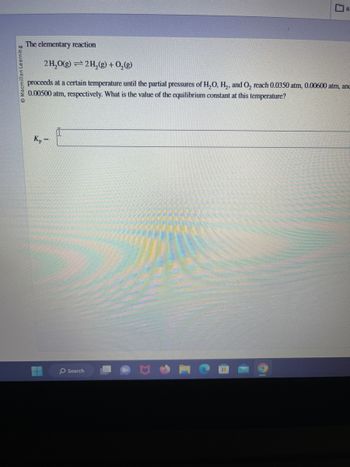
The elementary reaction
2H₂O(g) 2 H₂(g) + O₂(g)
=
Kp
proceeds at a certain temperature until the partial pressures of H₂O, H₂, and O₂ reach 0.0350 atm, 0.00600 atm, anc
0.00500 atm, respectively. What is the value of the equilibrium constant at this temperature?
=
R.
Search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In 20 words or less, please explain why the reaction conditions shown below are not ideal for saponification reactions and propose a solution to the problem. NaOH (cat), H₂O, Heat OH como earrow_forwardacid. b- 6. Consider the reaction below: (a). Highlight (or circle) the bonds that are broken/changed. (b). Identify the reaction type. (c). Explain why this makes Vitamin Ca good antioxidant. HO но Но—с-н Но-с-н ČH,OH ČH;OH !!! I 8 | 6 | OLarrow_forwardWhat is the product of the following reaction? excess KOH ?? heat OK OK -OK OK OK но. OK но но B D O B O A O Darrow_forward
- 60 50 $40 30 10 Course of Reaction Calculate the AH of the reaction. < Previous E Potential Energy (kJ)arrow_forwardI need help with this question!arrow_forwardConsider these two entries from a fictional table of standard reduction potentials. x2+ (aq) + 2e X(s) E = 2.25 V Y2+(aq) + 2e¯ Y(s) E = 0.31 V Which pair of species will react under standard conditions at 25 °C? OX and Y2+ x²+ and Y2+ contact us heip privacy policy terms of use about us careersarrow_forward
- Which reactant is limiting in a propane fire? O a) propane O b) charcoal O c) oxygen O d) water 99+ C F5 F6 F7 F8 F9 F10 F11 F12 & * 5 6 7 9. T Y U 团arrow_forwardBalance pleasearrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? NO+ Cu2+ NO₂ + Cut (reactant, product, neither) with a coefficient of. (Enter 0 for Water appears in the balanced equation as a neither.) How many electrons are transferred in this reaction?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY