
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
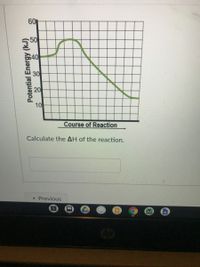
Transcribed Image Text:60
50
$40
30
10
Course of Reaction
Calculate the AH of the reaction.
< Previous
E
Potential Energy (kJ)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- e4bfa568b4fbef86cof575cbb Part D ?He+ °Pu 239 94 Express your answer as a no ΑΣφ TA chemical reaction does naarrow_forwardFor this problem, consider the following chemical reaction: 2 Na(s) + 2 HCl(aq) → 2 NaCl(aq) + H2(g) that follows the reaction energy diagram: Energyarrow_forwardThe equation is 4FeS(s) + 702(g ) -> 2Fe2O3(s) + 4SO2(g), the release is 251 kJ a) State whether reaction is exothermic or endothermic b ) Re-write the reaction with energy as a product or reactant (whichever is appropriate) c) Rewrite the reaction with the energy term written separately, to the right. d) Draw and label a potential energy diagram that describes the reactionarrow_forward
- 250 200 PE 150 (kJ) 100 50 ...... Reaction pathway Use the above potential energy diagram to answer the following question: What is the potential energy (PE) of the products?arrow_forwardSelect the potential energy diagram that corresponds to the decomposition of ozone (2 steps, overall exothermic) a Reaction Path с | b ll = 11 Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. d IV Reaction Path ||| Reaction Path IV Reaction Path Your answer Open in Reading Viarrow_forwardBased on the nature of reactants and products, which of the following processes would you expect to be exothermic? (You can select more than one). 2SO3 → 2S +30₂ N₂ + 3H₂ → 2NH₂ CO₂ → C+0₂ 2 NO ₂ → N₂O4 2arrow_forward
- Identify the bond(s) broken and the bond(s) formed in the reaction shown. Select all that apply. Br +Br₂ → H-Br formed (BDE 368 kJ/mol) C-H broken (BDE 381 kJ/mol) C-H broken (BDE 397 kJ/mol) C-Br formed (BDE 293 kJ/mol) C-Br formed (BDE 285 kJ/mol) C-Br formed (BDE 272 kJ/mol) Br-Br broken (BDE 193 kJ/mol) C-H broken (BDE 435 kJ/mol) HBrarrow_forwardWhat type of reaction and how much energy is involved in a reaction of boron trichloride with hydrochloric acid? B2Hog) +6Cl2ig) +>2BCl31g) + 6HClg) AH= -755.4 kJ/mole Endothermic with AH--755.4 kJ/mole. Endothermic with AH= +0.001324 kJ/mole. Endothermic with AH =+755.4 kJ/mole. Exothermic with AH=+755.4 kJ/mole. O Exothermic with AH--755.4 kJ/mole.arrow_forwardWhen solutions containing silver ions and chloride ions are mixed, silver chloride precipitates: Ag+ (aq) + Cl (aq)AgCl(s), AH=-65.5 kJarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY