
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:St. John's University - My App X
A ALEKS - Iffat Khan
G how many valence electrons in x
+
i www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-lijkPWvZoZLqkt1FLIq7wcPWKzBYGfE9IMFjNG0dB_IllkUmxjvdZ3p44elzs8GVwNxjbe5NR20.. *
O MEASUREMENT
Setting up the solution to a basic quantitative problem
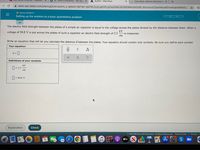
The electric field strength between the plates of a simple air capacitor is equal to the voltage across the plates divided by the distance between them. When a
voltage of 94.8 V is put across the plates of such a capacitor an electric field strength of 2.3
kV
is measured.
cm
Write an equation that will let you calculate the distance d between the plates. Your equation should contain only symbols. Be sure you define each symbol.
Your equation:
d =|
Definitions of your symbols:
kV
D = 2.3
cm
= 94,8 V
Explanation
Check
© 2021 McGraw-Hill Education. All Riahts Reserved. Terms of Use I Privacy
étv
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A tightly wound solenoid at 4.0 K is 50 cm long and is constructed from Nb wire of radius 1.5 mm. What maximum current can the solenoid carry if the wire is to remain superconducting?arrow_forwardUsing the given charge-to-mass ratios for electrons and protons, and knowing the magnitudes of their charges are equal, what is the ratio of the proton's mass to the electron's? (Note that since the charge-to-mass ratios are given to only three-digit accuracy, your answer may differ from the accepted ratio in the fourth digit.)arrow_forward(a) If the position of an electron in a membrane is measured to an accuracy of 1.00 m, what is the electron's minimum uncertainty in velocity? (b) If the electron has this velocity, what is its kinetic energy in eV? (c) What are the implications of this energy, comparing it to typical molecular binding energies?arrow_forward
- Check Your Understanding What happens to the ground state energy of an electron if the dimensions of the solid increase?arrow_forwardIn the simple Bohr model of the ground state of the hydrogen atom, the electron travels in a circular orbit around a fixed proton. The radius of the orbit is 5.281011m , and the speed of the electron is 2.18106m/s . The mass of an electron is 9.111031kg . What is the force on the electron?arrow_forwardCheck Your Understanding The vibrational frequency of the hydrogen iodide HI diatomic molecule is 6.691013Hz. (a) What is the force constant of the molecular bond between the hydrogen and the Iodine atoms? (b) What is the energy of the emitted photon when this molecule makes a transition between adjacent vibration energy levels?arrow_forward
- What is the critical magnetic field for lead at T = 2.8 K?arrow_forwardCheck Your Understanding If a=3+4i , what is the product a* a?arrow_forwardThe force on an electron is “negative the gradient of the potential energy function.” Use this knowledge and Equation 8.1 to show that the force on the electron in a hydrogen atom is given by Coulomb’s force law. Ur=ke2r(8.1)arrow_forward
- Measurements of a superconductor's critical magnetic field (in T) at various temperatures (in K) are given below. Use a line of best fit to determine Bc(0). Assume Tc = 9.3 K.arrow_forwardA section of superconducting wire carries a current of 100 A and requires 1.00 L of liquid nitrogen per hour to keep it below its critical temperature. For it to be economically advantageous to use a superconducting wire, the cost at cooling the wire must be less than the cost of energy lost to heat in the wire. Assume that the cost of liquid nitrogen is $0.30 per liter, and that electric energy costs What is the resistance of a normal wire that costs as much in wasted electric energy as the cost of liquid nitrogen for the superconductor?arrow_forwardFor an electron in a three-dimensional metal, show that the average energy is given by E=1N0EFEg(E)dE=35EF , Where N is the total number electrons in the metal.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning


University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning