
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
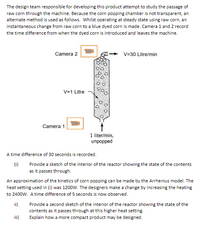
Transcribed Image Text:The design team responsible for developing this product attempt to study the passage of
raw corn through the machine. Because the corn popping chamber is not transparent, an
alternate method is used as follows. Whilst operating at steady state using raw corn, an
instantaneous change from raw corn to a blue dyed corn is made. Camera 1 and 2 record
the time difference from when the dyed corn is introduced and leaves the machine.
Camera 2
V=30 Litre/min
V=1 Litre
Camera 1
1 liter/min,
unpopped
A time difference of 30 seconds is recorded.
(i)
Provide a sketch of the interior of the reactor showing the state of the contents
as it passes through.
An approximation of the kinetics of corn popping can be made by the Arrhenius model. The
heat setting used in (i) was 1200w. The designers make a change by increasing the heating
to 2400w. A time difference of 5 seconds is now observed.
ii)
Provide a second sketch of the interior of the reactor showing the state of the
contents as it passes through at this higher heat setting.
iii)
Explain how a more compact product may be designed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- environment showing (b) Describe the mechanism of distillation operation in a chemical processing how McCabe -Thiele method can be used to determine the feed plate for a distillation column.arrow_forwardEstimate the cut diameter and overall collection efficiency of a cyclone given the particle size distribution containing algae. Particle size distribution and other pertinent data are given below. Average particle size dp, μm 1 5 10 20 30 40 50 60 >60 Wt % 3 20 15 20 16 10 6 3 7 Other design parameters are: Gas viscosity = 0.02 Cp; Specific Gravity of the particle = 3.0; Inlet gas velocity of cyclone = 14.6 m/s Effective number of turns within cyclone = 5 Cyclone diameter = 2.44 m Cyclone inlet width = 0.61 marrow_forward2. A 1 L, 100 g (dry weight) sample is removed from a continuous crystallizer which is operated with a residence time of 1000 s. The following particle size distribution is obtained for the sample: i) Size range (um) Mass (g) 200- 300- 400 500 600-700- 800- 300 400 500 600 700 800 900 0- 100- 100 200 2.5 26.0 30.0 21.0 12.0 6.0 2.0 0.5 0.0 Microscopy based on the sieve fractions has found that a volumetric shape correction factor, ky, of 0.52, for the crystal population and the crystal density has been found to be 2000 kg/m³ Determine whether the MSMPR model describes the system. If it does so, then determine the growth and nucleation rates for this system; if it does not, suggest why reasons that may cause deviation of this CSTR type crystallizer from the MSMPR model.arrow_forward
- Write an equation for a precipitation reaction and indicate when precipitation is thermodynamically feasible.arrow_forwardGive a table showing classification of mass transfer operations used in Chemical industries with phases of contactarrow_forwardDraw a diagram of the industrial sugar evaporation process in a multiple effect evaporator and explain its operation.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The