
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![The decomposition of N2O5 can be described by the equation
2N₂O₂(soln)
-
4 NO2 (soln) + O2(g)
Consider the data in the table for the reaction at 45 °C in carbon tetrachloride solution.
(s)
0
[N2O5] (M)
1.849
145
1.688
416
1.423
745
1.157
Given the data, calculate the average rate of reaction for each successive time interval.
What is the average rate of reaction for the time interval from 0s to 145 s?
1.11 ×10¯³
-3
average rate of reaction:
Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemistry | Publisher: University Science Books
M/s](https://content.bartleby.com/qna-images/question/1040cf11-1606-4441-95c2-42fee370d18a/1715ab81-f56d-4b4d-a731-e2118df678fc/3hw09jb_thumbnail.jpeg)
Transcribed Image Text:The decomposition of N2O5 can be described by the equation
2N₂O₂(soln)
-
4 NO2 (soln) + O2(g)
Consider the data in the table for the reaction at 45 °C in carbon tetrachloride solution.
(s)
0
[N2O5] (M)
1.849
145
1.688
416
1.423
745
1.157
Given the data, calculate the average rate of reaction for each successive time interval.
What is the average rate of reaction for the time interval from 0s to 145 s?
1.11 ×10¯³
-3
average rate of reaction:
Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemistry | Publisher: University Science Books
M/s
Transcribed Image Text:of 19
>
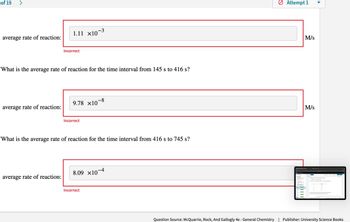
average rate of reaction:
1.11 ×10−3
Incorrect
What is the average rate of reaction for the time interval from 145 s to 416 s?
9.78 ×10-8
average rate of reaction:
Incorrect
What is the average rate of reaction for the time interval from 416 s to 745 s?
average rate of reaction:
8.09 ×10-4
Incorrect
> Attempt 1
M/s
M/s
123
Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemistry | Publisher: University Science Books
::
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The decomposition of hydrogen peroxide is described by the equation: 2H₂O₂ (aq)-2H₂O(0) + O₂(g) The reaction is first order in H₂O₂. It takes 10.0 hours for the concentration of H₂O₂ to drop from 2.0 M to 1.0 M. How many hours are required for the concentration of H₂O₂ to drop from 0.80 M to 0.050 M? 15.0 40.0 50.0 30.0 20.0 OOOOO Rarrow_forwardConsider this reaction: 2N₂O5 (g) → 2N₂O4 (g) + O₂(g) At a certain temperature it obeys this rate law. rate = (0.0317 M¹-s¯¹) [₂0₂] S Suppose a vessel contains N₂O5 at a concentration of 0.440 M. Calculate the concentration of N₂O5 in the vessel 480. seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M x10 ? X Sarrow_forwardConsider this reaction: 2N₂O5 (g) → 2N₂O4 (g) +O₂ (g) At a certain temperature it obeys this rate law. rate = (0.0328 s−¹)[N₂05] S Suppose a vessel contains N₂O5 at a concentration of 0.860M. Calculate the concentration of N₂O5 in the vessel 22.0 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M x10 X Śarrow_forward
- Consider following reaction: 2 A2B5(g) → 4AB2(g) + B2(g) The rate constant is 0.129 s-1 at 298 K for this reaction. What is the percent of A2B5 left after the first 19.091 s? Report your answer with three decimals. Enter numbers only;arrow_forwardA chemical engineer is studying the rate of this reaction. 2N,O; (g) → 2N,O4 (g) +O, (g) He fills a reaction vessel with N,O, and measures its concentration as the reaction proceeds. Here's a graph of his data: 0.8 0.6 0.4 0.2. 20 40 60 80 100 t (s) (W)['o°N]arrow_forwardThe oxidation of ammonia produces nitrogen and water via the following reaction: 4NH3(g) + 302(g) → 2N2(g) + 6H₂O(A) Suppose the rate of formation of H₂O(A) is 3.0 mol/(Ls). Which of the following statements is true? The rate of consumption of NH3 is 2.0 mol/(L. s). The rate of consumption of O₂ is 2.0 mol/(L. s). The rate of formation of N₂ is 1.3 mol/(Ls). The rate of formation of N₂ is 2.0 mol/(Ls). The rate of consumption of NH3 is 0.50 mol/(L. s).arrow_forward
- A possible reaction for the degradation of the pesticide DDT to a less harmful compound was simulated in the laboratory. The reaction was found to be first order, with k = 2.47 x 10-8 s-1 at 25°C. What is the half-life for the degradation of DDT in this experiment, in years? (1 year = 365 days)arrow_forwardA possible reaction for the degradation of the pesticide DDT to a less harmful compound was simulated in the laboratory. The reaction was found to be first order, with k = 2.55 x 108 s at 25°C. What is the half-life for the degradation of DDT in this experiment, in years? (1 year = 365 days) Round your answer to 2 decimal places.arrow_forwardThe overall reaction of ozone reacting to form oxygen has been proposed to occur in a reaction mechanism of: Step 1: O3(g)-→O2(g) + O(g) Step 2: O3(g) + O(g) →202(g) What is the role of O(g) and what is the overall balanced equation? Periodic Table and Datasheet Select one: O a. O(g) is an intermediate; 203(g) + O(g) →302(g) + O(g) O b. O(g) is a catalyst; 203(g) + O(g)→302(g) + O(g) O c. O(g) is an intermediate; 203(g) → 302(g) O d. O(g) is a catalyst; 203(g)→302(g) Which statement best describes general equilibrium?arrow_forward
- I completed the problem but for some reason, my answer is still wrong. for part a I got 5.48 x 10^-5 and for part b I got 5.61x10^-4. I substituted the concentrations in the equation but hey still seem to be wrong can someone explain?arrow_forwardConsider this reaction: →NH4OHaq+NH3aqH2Oaq At a certain temperature it obeys this rate law.rate =·26.0M−1s−1NH4OH2 Suppose a vessel contains NH4OH at a concentration of 1.28M. Calculate the concentration of NH4OH in the vessel 0.270 seconds later. You may assume no other reaction is important.Round your answer to 2 significant digits. =___Marrow_forwardAt a certain temperature the rate of this reaction is first order in H₂CO3 with a rate constant of 4.73 s H₂CO3(aq) → H₂O (aq) + CO₂ (aq) Suppose a vessel contains H₂CO3 at a concentration of 1.01 M. Calculate how long it takes for the concentration of H₂CO3 to decrease to 0.212M. You may assume no other reaction is important. olo Round your answer to 2 significant digits. B s ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY