
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
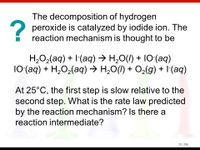
Transcribed Image Text:The decomposition of hydrogen
2 peroxide is catalyzed by iodide ion. The
reaction mechanism is thought to be
H,O2(aq) + I(aq) → H,0(I) + 10(aq)
10(aq) + H,O2(aq) → H,O(I) + O2(g) + I(aq)
At 25°C, the first step is slow relative to the
second step. What is the rate law predicted
by the reaction mechanism? Is there a
reaction intermediate?
13 | 106
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student obtained the following data for the gas phase decomposition of hydrogen peroxide at 400 °C. H₂O₂(g) H₂O(g) + 2 O₂(g) [H₂0₂], M seconds 0.134 0 6.70x10-² 14.2 ◊ 3.35x10-2 42.7 (1) What is the half-life for the reaction starting at t=0 s? What is the half-life for the reaction starting at t=14.2 s? Does the half-life increase, decrease or remain constant as the reaction proceeds? 1.68x10-2 99.2 (2) Is the reaction zero, first, or second order? (3) Based on these data, what is the rate constant for the reaction? S M-¹S-1arrow_forwardWhen designing a consumer product, it is desirable for it to have a two-year shelf life. Often this means that the active ingredient in the product should not decrease by more than 5% in two years. If the reaction is first order, and the rate constant is: k = 2.56 × 10-2 yr 1, determine the half-life of an active ingredient that has a shelf life of 2.00 years. The half-life of an active ingredient is i yr.arrow_forwardSuppose the formation of dinitrogen pentoxide proceeds by the following mechanism: step elementary reaction 1 NO₂ (g) +03 (g) → NO3 (g) + O₂(g) 2 NO3 (g) + NO₂ (g) → N₂O5 (g) Suppose also k₁ « k₂. That is, the first step is much slower than the second. Write the balanced chemical equation for the overall chemical reaction. Write the experimentally- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. rate = k rate constant k₁ k₂ ロ→ロ X Śarrow_forward
- The rate equation for the hydrolysis of sucrose to fructose and glucose C₁2H₂O(aq) + H₂O()→ CsH₁2Os(aq) + CH₂Os (aq) is-A[sucrose]/At=k[C₁₂H₂O₂). After 28 minutes at 27 °C, the sucrose concentration decreased from 0.0112 M to 0.0090 M. Find the rate constant k. k= min-1 Submit Answer Try Another Version 4 item attempts remainingarrow_forwardThe following data were obtained for the reaction of carbon monoxide with chlorine, producing phosgene, COCl2, a highly toxic gas CO(g) + Cl2(g) → COCl2(g) From the following data, obtained at 360 K, determine: a) The order of the reaction, and the reaction rate law b) The value of the reaction rate constant, k c) The initial reaction rate for the formation of COCl2 when the concentration of CO is 0.30 M and that of Cl2 is 0.40 M The following data were obtained for the reaction of carbon monoxide with chlorine, producing phosgene, COCl2, a highly toxic gas CO(g) + Cl2(g) → COCl2(g) From the following data, obtained at 360 K, determine: a) The order of the reaction, and the reaction rate law b) The value of the reaction rate constant, k c) The initial reaction rate for the formation of COCl2 when the concentration of CO is 0.30 M and that of Cl2 is 0.40 M The following data were obtained for the reaction of carbon monoxide with chlorine, producing phosgene, COCl2, a highly toxic…arrow_forwarda)A nickel catalyst is commonly used in the hydrogenation of ethylene. If the initial concentration of ethylene is 2.75 mol·L−1 and the rate constant for the reaction is 0.0018 mol·L−1·s−1, what is the rate of reaction if it follows a zero-order reaction mechanism? Express your answer to two significant figures. b)Determine the half-life for the reaction in Part B. Express your answer to two significant figures.arrow_forward
- Consider the following reaction: 4 HBr(g) + O2(g) 2 H2O(g) + 2 Br2(g)(a) The rate law for this reaction is first order in HBr(g) and first order in O2(g). What is the rate law for this reaction?(b) If the rate constant for this reaction at a certain temperature is 8.80e+03, what is the reaction rate when [HBr(g)] = 0.00429 M and [O2(g)] = 0.00758 M?Rate = _______ M/s.(c) What is the reaction rate when the concentration of HBr(g) is doubled, to 0.00858 M while the concentration of O2(g) is 0.00758 M?Rate = _______ M/sarrow_forwardChlorine monoxide (ClO) accumulates in the stratosphere above Antarctica each winter and plays a key role in the formation of the ozone hole above the South Pole each spring. Eventually, ClO decomposes according to the equation: 2C1O (g) → Cl2 (g) + O2 (g) The kinetics of this reaction were studied in a laboratory experiment at 298K, and the data are shown in the table below. (a) Determine the order of the reaction (b) Determine the rate law expression (c) Determine the value of the rate constant, k, at 298K. Time (ms) [CiO] (M) In [ClO] cioj (M-1) [CiO] 6.67 × 107 1.39 x 108 -8- 1.50 x 10 7.19 x 10 4.74 × 10 -18.0 6- 10 -18.8 6- 20 -19.2 2.11 x 108 6- 30 3.52 х 10° -19.5 2.84 x 108 6- 40 2.81 x 10 -19.7 3.56 x 108 6- 100 1.27 x 10 -20.5 7.87 x 108 10 200 6.60 x 10- -21.1 1.52 x 10°arrow_forwardA possible reaction for the degradation of the pesticide DDT to a less harmful compound was simulated in the laboratory. The reaction was found to be first order, with k = 2.47 x 10-8 s-1 at 25°C. What is the half-life for the degradation of DDT in this experiment, in years? (1 year = 365 days)arrow_forward
- Consider these three reactions as the elementary steps in the mechanism for a chemical reaction.(i) Cl2 (g) + Pt (s) à 2Cl (g) + Pt (s) Ea = 1550 kJ ∆H = – 950 kJ(ii) Cl (g)+ CO (g) + Pt (s) à ClCO (g) + Pt (s) Ea = 2240 kJ ∆H = 575 kJ(iii) Cl (g) + ClCO (g) à Cl2CO (g) Ea = 2350 kJ ∆H = – 825 kJ e. Which reaction intermediate would be considered a catalyst (if any) and why?f. If you were to add 2700kJ of activation energy to the reaction, would you be able to make thereaction reverse itself (i.e. have the products become reactants)? Justify your answer.g. If you were to added a positive catalyst to step (iii) what would the end result be? Justify yourprediction.h. Your friend is looking at your graph and states that she believes that step (ii) is the ratedetermining step. Do you agree with her? Justify your reasoning.arrow_forwardPlease and thank you ?arrow_forwardThe following reaction was studied at 25°C. The following results were obtained. a) What is the rate law for this reaction at this temperature? b) What is the value of the rate constant? Be sure to include the correct units in your answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY