
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
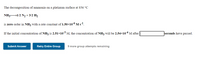
Transcribed Image Text:The decomposition of ammonia on a platinum surface at 856 °C
NH31/2 N2 + 3/2 H2
is zero order in NH3 with a rate constant of 1.50×10-6 M s-1.
If the initial concentration of NH, is 2.51x10-3 M, the concentration of NH3 will be 2.54×10-4 M after
seconds have passed.
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Need help with this question. Thank you :)arrow_forwardThe activation energy for the gas phase isomerization of methyl cis-cinnamate is 174 kJ/mol.cis-C6H5CH=CHCOOCH3trans-C6H5CH=CHCOOCH3The rate constant for this reaction is 5.94×10-4 s-1 at 660 K. What is the rate constant at 707 K?Answer: ____ s-1arrow_forwardDecomposition of lead(II) nitrate gives lead(II) oxide, nitrogen dioxide gas, and oxygen gas. How many moles of oxygen gas form when 275.0 g lead(II) nitrate completely decomposes? ☐0.275 ☐0.391 ☐0.415 ☐0.662 ☐none of themarrow_forward
- < 2:19 A) S-¹ B) M S-¹ What are the units for the rate constant of a first-order reaction? C) M-¹ s¹ D) M-² S-¹ Question 11 of 15 E) s M-¹ 5GW Tap here or pull up for additional resources Submitarrow_forwardI don’t know how to find the activation energy in this problemarrow_forwardnst-order reaction At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.00555 s 250, (g) 2S0, (9) +0, (g) Suppose a vessel contains SO, at a concentration of 0.360 M. Calculate the concentration of SO, in the vessel 130. seconds later. You may assume no other 3 reaction is important. db Round your answer to 2 significant digits. OM Explanation Check 2021 McGraw-Hill Education. All Rights Reserved Terms of Use Privacy Accessibility O v D 9:42 M acer pagarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY