
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The decomposition of [A] in solution at 80 °C proceeds via the following reaction:
A (aq) → B (aq)
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results. What is the energy of activation (kJ/mol) for this reaction?
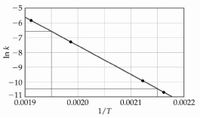
Transcribed Image Text:-5
-6
-7
-9
- 10
-11
0.0019
0.0020
0.0021
0.0022
1/T
In k
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine which of the two diagrams here (both for the same reaction) involves a catalyst, and identify the activation energy for the catalyzed reaction: 100 100 90 80 70 60 90 80 70 60 50 40 30 50 40 30 20 10 20 10 Extent of reaction Extent of reaction (a) (b) Energy (kJ) Energy (kJ)arrow_forwardA researcher raises the temperature from 47.4 to 64.7 °C and finds that the rate of the reaction doubles. What was the activation energy (in Joules) for this reaction? (R = 8.3145 J/molk)arrow_forwardA researcher raises the temperature from 39.4 to 56.2 o C and finds that the rate of the reaction doubles. What was the activation energy (in J/mol) for this reaction? (R = 8.3145 J/mol K)arrow_forward
- The activation energy, Ea, for a particular reaction is 13.6 kJ/mol. If the rate constant at 754 °C is 24.5/min, at what temperature (in °C) will the rate constant be 17.5/min? (R = 8.314 J/mol • K)arrow_forwardThe energy of activation for a particular reaction is 23.7 kJ/mol. At 16.3 °C the rate constant of the reaction is 3.38 × 10° h. 1 Calculate the temperature (in °C) when the rate constant, k = 1.62 × 10 h. 1 R = 8.314472 J/mol K. Report your answer to THREE significant figures.arrow_forwardThe activation energy, Ea, for a particular reaction is 19.4 kJ/mol. If the rate constant at 80 °C is 0.820 M⁻¹s⁻¹, then what is the value of the rate constant at 149 °C? (R = 8.314 J/mol • K)arrow_forward
- The activation energy, Ea, for a particular reaction is 19.4 kJ/mol. If the rate constant at 80 °C is 0.820 M⁻¹s⁻¹, then what is the value of the rate constant at 179 °C? (R = 8.314 J/mol • K)arrow_forwardThe activation energy, Ea, for a particular reaction is 37.8 kJ/mol. If the rate constant at 280 K is 0.178 M/s, then what is the value of the rate constant at 391 K? (R = 8.314 J/mol • K)arrow_forwardThe activation energy, Ea, for a particular reaction is 19.4 kJ/mol. If the rate constant at 80 °C is 0.820 M⁻¹s⁻¹, then what is the value of the rate constant at 249 °C? (R = 8.314 J/mol • K)arrow_forward
- The activation energy, Ea, for a particular reaction is 13.6 kJ/mol. If the rate constant at 754 °C is 24.5/min, at what temperature (in °C) will the rate constant be 17.9/min? (R = 8.314 J/mol • K)arrow_forwardThe activation energy, Ea, for a particular reaction is 37.8 kJ/mol. If the rate constant at 280 K is 0.178 M/s, then what is the value of the rate constant at 417 K? (R = 8.314 J/mol • K)arrow_forwardThe activation energy, Ea, for a particular reaction is 19.4 kJ/mol. If the rate constant at 80 °C is 0.820 M⁻¹s⁻¹, then what is the value of the rate constant at 333 °C? (R = 8.314 J/mol • K)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY