Question
thumb_up100%
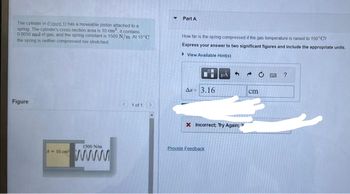
Transcribed Image Text:The cylinder in (Eigure 1) has a moveable piston attached to a
spring The cylinder's cross-section area is 10 cm² it contains
0 0050 mol of gas, and the spring constant is 1500 N/m. At 15°C
the spring is neither compressed nor stretched
Figure
A-10 cm
1500 N/m
www
1 of 1
Y
Part A
How far is the spring compressed if the gas temperature is raised to 150°C?
Express your answer to two significant figures and include the appropriate units.
View Available Hint(s)
Ar 3.16
μA
Provide Feedback
4
X Incorrect; Try Again;
→
cm
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- N2 gas in a container is contracted by one third of the original volume to a total of 12 dm3 isothermally. The pressure is now 2 bar, what was the original pressure before the contraction?arrow_forward5.0 g of nitrogen gas at 20°C and an initial pressure of 2.8 atm undergo a constant-pressure expansion until the volume has tripled. Part A What is the gas volume after the expansion? Express your answer with the appropriate units. ► View Available Hint(s) V= Submit HA Value Previous Answers Units ? X Incorrect; Try Again; 5 attempts remainingarrow_forwardR Review I Constants Part A A container holds 1.0 g of argon at a pressure of 8.0 atm. How much heat is required to increase the temperature by 100° C at constant volume? Express your answer with the appropriate units. H HẢ Q = Value Units %3D Submit Request Answer Part B How much will the temperature increase if this amount of heat energy is transferred to the gas at constant pressure? Express your answer in kelvins. ΑΣφ K AT =arrow_forward
- The image shows a cylinder with a movable wall (partition) and a piston. Movable partition Piston -Work Vacuum Gas What properties of matter allow the gas to expand and contract as the wall is removed and the piston moved as depicted in the image? O A The particles of a gas are close together, they completely fill the volume of the container, they are strongly attracted to each other. ©2021 Illuminate Education TM, Inc. hp -> esc #3 %24 96arrow_forwardA large cylindrical tank contains 0.750 m³ of nitrogen gas at 22.0°C and 6.50x103 Pa (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. Part A What will be the pressure if the volume is decreased to 0.530 m³ and the temperature is increased to 165°C? Express your answer in pascals. —| ΑΣΦ P = Submit Request Answer < Return to Assignment Provide Feedback ? Paarrow_forwardA 50 L steel tank full of gasoline at -20 C. What is the volume difference for both tank and fuel at 40C. What is the overflow?arrow_forward
- week 4 #4 What is the total translational kinetic energy of the gas molecules of air at atmospheric pressure that occupies a volume of 1.40 L? _______ Jarrow_forward0.50 mol of argon gas is admitted to an evacuated 50 cm³ container at 20°C. The gas then undergoes an isobaric heating to a temperature of 340 °C. Part A What is the final volume of the gas? Express your answer with the appropriate units. V₁ = Submit HÅ Value Request Answer Unitsarrow_forward70 J of work are done on the gas in the process shown in (Figure 1). Figure p (kPa) 300 200 100- Screenshot f i 0 V₁ 2V₁ 3V₁ 1 of 1 V Part A What is V₁ in cm³? Express your answer in cubic centimeters. ► View Available Hint(s) V₁ = Submit VT ΑΣΦ Provide Feedback B] ? cm³arrow_forward
arrow_back_ios
arrow_forward_ios