
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A 50 L steel tank full of gasoline at -20 C. What is the volume difference for both tank and fuel at 40C. What is the overflow?
Expert Solution
arrow_forward
Step 1
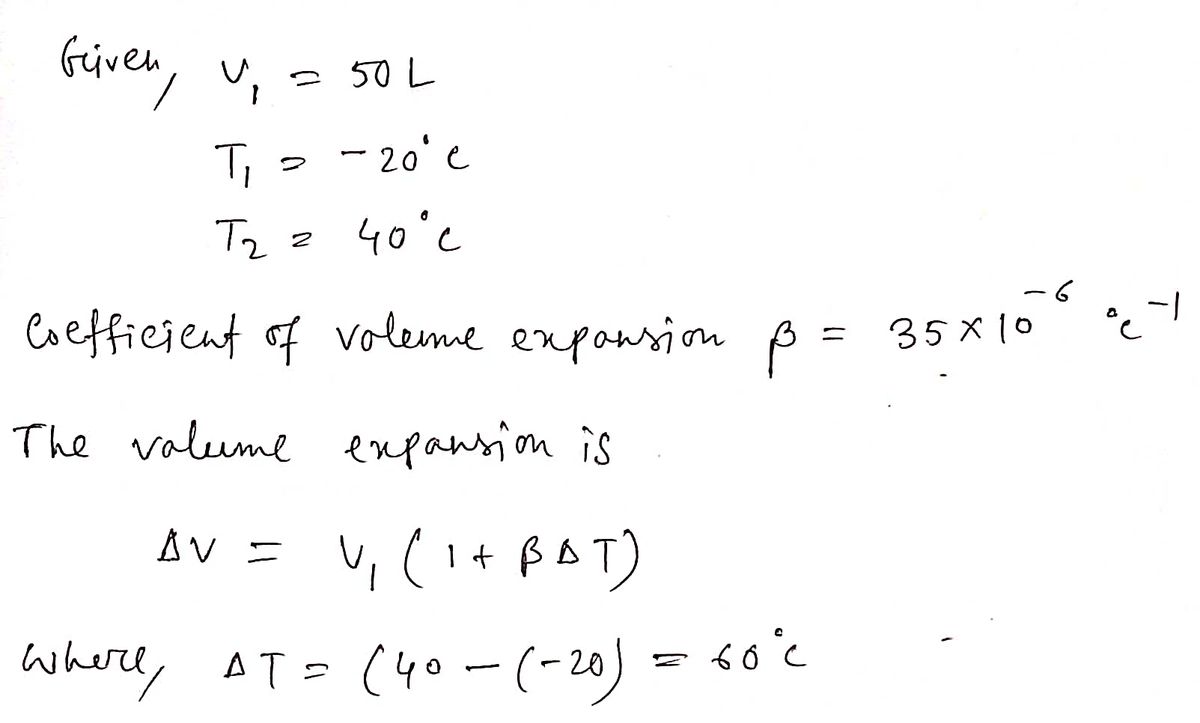
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What is the RMS speed of CO2 gas at a temperature of 270 C? a. 372 m/s b. 505 m/s c. 268 m/s d. 305 m/s e. 412 m/sarrow_forwardWhat is the absolute pressure of the air in your car’s tires, in psi, when your pressure gauge indicates they are inflated to 35.0 psi? Assume you are at sea level.arrow_forwardThe density of mercury is 13 600 kg/m3 at 0 oC. What would be its density at 151 oC?arrow_forward
- A rocket engine shoots exhaust gas at 5.0 EH PA of gas pressure through a nozzle who's area is 0.3 M to what is the fourth pushing the rocket forward?arrow_forward19. The radius of the bronchial tube is decreased by a factor of 4. The velocity of gas through it initially is 1 m/s. What is the velocity in the section of bronchial tube with decreased radius in m/s?arrow_forwardWhat is gas density, if under pressure of 105 Pa, its molecules move at a speed of 300 m/s? /Give the answer in [kg/m3] /arrow_forward
- V3arrow_forwardThe density of air at 20 degree celcius is 1.204 kg/m3. The density at temperature 100 degree celcius is 0.9467 kg/m3. If an average size hot air balloon envelopes a volume of 2200 m3, how much weight can it lift (including the balloon itself)? a. 5.55 kN b. 11.1 kN c. 20.4 kN d. 26.0 kNarrow_forwardI put air in the tires of my car during the summer, while it was 28.8 °C outside (about 83.8 °F). My tires say to fill them to a pressure of 203,000 Pa. Boltzmann's constant is 1.38 * 10 ^ - 23 * J / K A) If my tires have a volume of 0.0100 m³, how many air molecules are in the tire? N = molecules B) These tires go all summer without losing any air, however come fall the temperature drops down to 8.65 °C (about 47.6 °F). Assuming the volume doesn't change (because it doesn't) what is the new pressure in my tires? P=arrow_forward
- 1. Five bicyclists are riding at speeds 2.6 m/s, 4.7 m/s, 5.4 m/s, 3.5 m/s, and 2.6 m/s. a) What is their average speed? V ave b) What is their rms speed? Vrms m/s m/sarrow_forwardThe absolute pressure of a gas in an enclosed cylinder on a day is 3.67 A low pressure storm comes upthe conditions in the gas cylinder remain unchanged does the absolute pressure of the gas increase, decrease, or remain the same? What about the gauge pressure ?arrow_forwardIt is known that 28 g of a certain ideal gas occupy 26.4 liters at standard conditions (0° C, 1 atm). The volume occupied by 42 g of this gas at standard conditions (in liters) is: a. 22.4 b. 14.9 c. 33.4 d. more data are needed e. 39.6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON