
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Profiles Tab Window Help
Paraph
cics....pdf
Gramm
nment/takeCovalent Activity.do?locator=assignment-take
Su
My Ho OV X
A
Common Greek(upper)
X⁰ XX
Undo Redo Clear Help
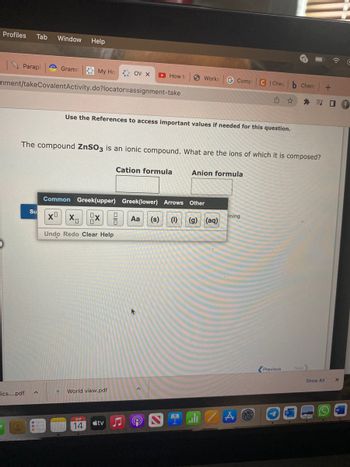
The compound ZnSO3 is an ionic compound. What are the ions of which it is composed?
Use the References to access important values if needed for this question.
World view.pdf
14
How to
tv
Cation formula
DIC
Greek(lower)
Works G Comp
Anion formula
Arrows Other
Aa (s) (1) (g) (aq)
ali
C Cheg
u
ining
Chem +
★河口
Previous
Next
Show All
A
X
с
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OWLV2 | Online teachin X According To The Following+ My Home gagenow.com/ilm/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAsignmentSessionLocator=assignment-take [Review Topics] [References] E 2req Use the References to access important values if needed for this question. 3 2reg For the following reaction, 0.397 grams of hydrogen gas are allowed to react with 54.1 grams of iodine. S 2req hydrogen(g) + iodine(s) – → hydrogen iodide(g) What is the maximum mass of hydrogen iodide that can be formed? grams What is the FORMULA for the limiting reagent? Es 2req is 2req What mass of the excess reagent remains after the reaction is complete? grams ts 2req ts 2req Submit Answer Retry Entire Group 9 more group attempts remaining ts 2req ots 2req ots 2reg ots 2req ots 2req pts 2req Previor Show Hint a $ hp Ins prt sc f9 12 delete 16arrow_forward8. Acids combine in a similar way as ionic compounds. Write the formula of the compound formed by combining the following ions. Use the ion cut outs to help as needed. F- SO 2- PO43- H* 161olrb mublearrow_forwardWrite the empirical formula for at least four ionic compounds that could be formed from the following ions: NH4, Fe³+, C₂H₂0₂, MnO4 0 0.0....arrow_forward
- Assign partial charges (8+ or 8-) to each atom indicated by an arrow. If an atom has no partial charge indicate this by writing 'no charge' by the appropriate arrow. :CI: 0: O: + :NEC -N-H H H H H H (a) (b) (d) (e) (f)arrow_forwardOnly typed solutionarrow_forwardRow 2 and 3 are correct, need help with row 1arrow_forward
- Which of the following is the correct Lewis structure for OH"? •0-H =H -H- [#一 1O M DELLarrow_forward€ Name the fouowing compounds NH, C! Si BG Mnz (6os)s Covalent - compound roith 2 non - metals jonic compound oith non-metal AND metal so N203arrow_forwardGive detailed Solution (no need Handwritten answerarrow_forward
- Calculate the mass of 5.62 x 1024 molecules of an unknown covalent compound. (molar mass 180 g/mol) O 1.81 x 1023 g O 1.68 x 10 g O 1.21 x 1046 g 1.89 garrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 3+ Co 2+ Fe Continue Cu anion 3- PO co C103 Some ionic compounds empirical formula name of compound Xarrow_forwardWe've spent quite a bit of time thinking about the interactions between atoms or molecules, one of which is called London dispersion forces. The stable interactions between two atoms we briefly discussed, in this case a covalent bond. We will explore more about this type of interaction in a minute. But let's say for now that it takes 348 kJ/mol to break 1 mole of C-C bonds (that is, 6.022 * 1023 C-C bonds). What would be the minimum frequency of a photon that could break a single (1) C-C bond? What region of the EM spectrum would this light be in? Enter your answer herearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY