
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
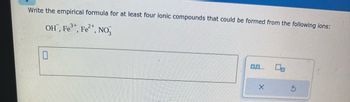
Transcribed Image Text:Write the empirical formula for at least four ionic compounds that could be formed from the following ions:
OH, Fe³+, Fe²+, NO3
0
0.0....
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation anion 3+ Fe 2+ Fe Cs Sp. 2+ CI CI CI CI Some ionic compounds empirical formula 00 name of compound 0 X 00arrow_forwardConsider these compounds: A. Ag2SO3 B. MnS C. Mg(OH)2 D. FeCO3 Complete the following statements by entering the letter(s) corresponding to the correct compound(s). (If more than one compound fits the description, include all the relevant compounds by writing your answer as a string of characters without punctuation, e.g, ABC.) Without doing any calculations it is possible to determine that cobalt(II) carbonate is more soluble than cobalt(II) carbonate is less soluble than It is not possible to determine whether cobalt(II) carbonate is more or less soluble than comparing Ksp values. I and by simplyarrow_forwardConsider these compounds: A. MNCO3 B. PbCO3 C. Ag,SO3 D. PbI, Complete the following statements by entering the letter(s) corresponding to the correct compound(s). (If more than one compound fits the description, include all the relevant compounds by writing your answer as a string of characters without punctuation, e.g. ABC.) Without doing any calculations it is possible to determine that manganese(II) hydroxide is more soluble than and manganese(II) hydroxide is less soluble than It is not possible to determine whether manganese(II) hydroxide is more or less soluble than by simply comparing Kap values.arrow_forward
- 8.6 x 10²³ formula units of NaCl is equal to how many moles H?arrow_forwardWrite the empirical formula for at least four ionic compounds that could be formed from the following ions:arrow_forwardWrite the empirical formula for at least four ionic compounds that could be formed from the following ions:arrow_forward
- Calculate the total number of ions in 8.4 moles of CaCl2. 1.5 × 1025 ions 5.0 × 1024 ions 1.7 × 1027 ions 2.2 × 1029 ionsarrow_forwardGive detailed Solution (no need Handwritten answerarrow_forwardWrite the empirical formula for at least four ionic compounds that could be formed from the following ions:CH3CO−2,ClO−3, Fe2+, Pb4+arrow_forward
- Write the empirical formula for at least four ionic compounds that could be formed from the following ions: PO, NH, I03, Fe2- Fe²* 4 ! (NH,), (PO,), O (РО, 4 0,0,...arrow_forwardCalculate the mass of 5.62 x 1024 molecules of an unknown covalent compound. (molar mass 180 g/mol) O 1.81 x 1023 g O 1.68 x 10 g O 1.21 x 1046 g 1.89 garrow_forwardHow many moles of UF6 would have to be decomposed to provide enough fluorine to prepare 2.06 mol of CF4? (Assume sufficient carbon is available.) i mol UF6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY