
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
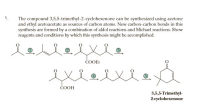
Transcribed Image Text:The compound 3,5,5-trimethyl-2-cyclohexenone can be synthesized using acetone
and ethyl acetoacetate as sources of carbon atoms. New carbon-carbon bonds in this
synthesis are formed by a combination of aldol reactions and Michael reactions. Show
reagents and conditions by which this synthesis might be accomplished.
lel
COOEt
exelte.
ČOOH
3,5,5-Trimethyl-
2-cyclohexenone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 4. Draw the organic products of the reactions of (R)-2-iodobutane with azide ion and methanol. Draw allstereoisomers expected to form. (R)-2-iodobutane Na*N3* CH3OHarrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. aqueous H₂SO + KCNarrow_forward1. NaOEt CO₂Et 2. H3O+ 3. heat Br H3C CO₂H CO2 H3C CO₂Et The malonic ester synthesis is a carbonyl alkylation reaction. It is used to prepare carboxylic acids from primary alkyl halides, lengthening the carbon chain by two atoms. Thus, the product can be visualized as being a "substituted acetic acid." The reaction consists of three steps: generation of the enolate anion followed by SN2 reaction with a primary alkyl halide, ester hydrolysis under acid conditions, and decarboxylation. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions 22 CX EtO₂C EtO₂C HOCH2CH3 HOCH2CH3 EtO₂C EtO₂Carrow_forward
- онarrow_forward4. Using resonance structures, show how the intermediate is stabilized in the reaction of p-chloronitrobenzene with pyrrolidine in the substitution reaction shown: NO 2 NO2 H N. N CI +arrow_forward2-Pentanol reacts with hydrochloric acid to undergo a substitution reaction. What is the mechanism for this, showing all the possible products. However, if 2- butanol reacts with p-toluene sulfonic acid (p-TSA) only the elimination product is formed. No substitution product is observed. Why? Identify the possible products for this reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY