
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
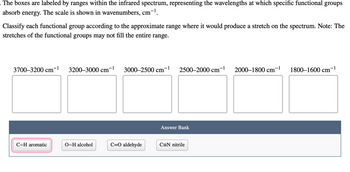
Transcribed Image Text:The boxes are labeled by ranges within the infrared spectrum, representing the wavelengths at which specific functional groups absorb energy. The scale is shown in wavenumbers, cm⁻¹.
Classify each functional group according to the approximate range where it would produce a stretch on the spectrum. Note: The stretches of the functional groups may not fill the entire range.
- 3700–3200 cm⁻¹
- 3200–3000 cm⁻¹
- 3000–2500 cm⁻¹
- 2500–2000 cm⁻¹
- 2000–1800 cm⁻¹
- 1800–1600 cm⁻¹
**Answer Bank:**
- C–H aromatic
- O–H alcohol
- C=O aldehyde
- C≡N nitrile
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- question attached:arrow_forwardLook at the three infrared spectra in Figures C to E and answer the following questions (a) Are any of the spectra that of an alcohol? If so, which? What absorption pattern(s) at what wavelength(s) identifies an alcohol? (b) Are any of the spectra that of a compound containing a benzene ring? If so, which? What three absorption patterns at what wavelengths show that a compound has a benzene ring? (c) Are any of the spectra that of a compound containing only carbons and hydrogens? If so, which? Benzene rings contain only carbons and hydrogens. Might the spectrum or spectra you chose for your answer above indicate a benzene ring? (Tell what absorption patterns are present or not present that would support your answer.)arrow_forwardUsing the information provided, list the approximate wavenumber ranges corresponding to the bands that you think are from C-H and O-H stretching (no need to distinguish which is which at this point). Which bands do you think result from different C-C and C-O stretching and bending vibrations? C-H and O-H: C-C:arrow_forward
- Bb Microsoft PowerPoi X A molecule with the molecular formula of CsH10O produces the IR spectrum shown below. Draw a structure that best fits this data. Bb Microsoft Pow X ← → с app.101edu.co ← Microsoft PowerPoi X Bb Microsoft PowerPoi X 3000 Microsoft Pow X app.101edu.co 3000 2000 [a] A molecule with the molecular formula of C7HBO produces the IR spectrum shown below. Draw a structure that best fits this data. Wavnumbers") Bb Microsoft Pow: X Nucleophilic Substi X Nucleophilic: Problem 22 of 16 X Aktiv Chemistry Atoms, Bonds and Rings Draw or tap a new bond to see suggestions. Aktiv Chemistr X Problem 24 of 16 Atoms, Bonds and Rings Charges X b Search results X Charges C Post a new questio Ⓒo X CA molecule wi X + + B Undo Remove Undo Remove ★ I Reset Ⓒ Done Reset * ☐ Ⓒ Done T Drag To Pan x Submit ⠀ ★ T I Drag To Pan F Submitarrow_forwarda) Propose structures for compounds of the following molecular formulas, using the spectra given below.Show your workarrow_forwardMatch the following IR spectrum with the correct molecule.arrow_forward
- Considering IR spectroscopy, which of the following statements is incorrect? options: Concentrated alcohols give rise to broad signals while dilute alcohols give rise to narrow signals Carboxylic acids exist as dimer due to hydrogen bonding and thus produce a narrow signal at 3600 cm-1 and a characteristic signal due to C=O at 1700 cm-1 C=O bonds produce strong signals in an IR spectrum while C=C bonds often produce a weak signals Symmetrical C=C bonds and C≡C bonds do not produce signals Primary amines produce two signals resulting from symmetric stretching and asymmetric stretchingarrow_forwardThe simulated APT spectrum of a compound with the molecular formula C6H12 is shown. Draw a structure that is consistent with this data.arrow_forwardHow do I interpret, label, and determine which molecule this H NMR graph represents? The three molecule choices are methylene cyclohexane, 3-methylcyclohexene, or 1-methylcyclohexene. (the two pictures of the H NMR graph is from the same graph)arrow_forward
- Based on the spectra provided, draw the structure. Label each unique carbon and hydrogen with the letters A, B, C… for use in assigning NMR peaks. fill in the data table assigning peaks in each spectrum. You should assign: • All 1H NMR peaks • Significant IR peaks above 1600 cm Example of what the table should include (imagine the structure of ethanol is drawn with the CH3 hydrogens labeled as A, the CH2 hydrogens labeled as B, and the OH hydrogen labeled as C) : hydrogen proton chemical shift integration splitting pattern couples to.. A 1.2 3 triplet B B 3.7 2 quartet A C 2.6 1 singlet -arrow_forwardWhy do aldehydes, esters, and amides all have a strong absorption in the 1630-1780 cm1 region of their IR spectra? A) The bond between H and the sp³-hybridized C in these functional groups vibrates in this energy range. B) Each of these functional groups has at least two resonance structures, and the different vibrations of the resonance structures give off energy in this region. C) The bond between O and the sp²-hybridized C in these functional groups vibrates at a frequency in this energy range. D) Light at this wavenumber causes the average C to O bond length to increase which causes more of this light to be transmitted. E) An electron in the bond of these functional groups gets excited to the * orbital.arrow_forwardEach of the two IR spectra are one of the following benzene derivatives: benzaldehyde, benzoic acid, benzyl alcohol, methyl benzoate, and benzamide. What derivative is represented by each graph?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY