
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Temperatures (°C) are measured at various points on a heated plate, which are reported in the following table. Estimate the temperature when X=4 and Y=3.2, X=4.3 and Y=2.7 by Lagrange polynomial.
Image
Help with this problem please, we are working using only X and Y data, but never anything more than this so I don't know how to apply the formulas when we are given more data and I am confused for this task.
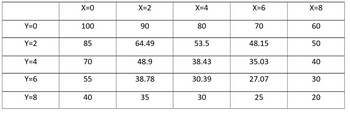
Transcribed Image Text:Y=0
Y=2
Y=4
Y=6
Y=8
X=0
100
85
70
55
40
X=2
90
64.49
48.9
38.78
35
X=4
80
53.5
38.43
30.39
30
X=6
70
48.15
35.03
27.07
25
X=8
60
50
40
30
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 14 images

Knowledge Booster
Similar questions
- The rate of heat transfer from a body to the surroundings is governed by the following equation: D= hA(Tsur - Tbody) Where dis the heat transfer rate (J/s) h is the heat transfer coefficient A is the heat transfer area (m²) Tsur is the temperature of the surroundings (K) Tpody, is the temperature of the body (K) Find the units for the heat transfer coefficient, h.arrow_forwardthe barometer shows a value of 740 mm Hg; dry bulb temperature 35 ° C; wet bulb temperature is 25 ° C. Specify:a. Relative humidityb. Absolute humidityc. Dew point temperaturearrow_forwardSelect all statements that apply to constant volume calorimetry. A coffee cup calorimeter is used. A bomb calorimeter is used. The system is the reaction or process. The system is the water plus the calorimeter. The surroundings include the reaction or process only. The surroundings include the water plus the calorimeter. The temperature of the water will always increase. The temperature of the water will always decrease. The temperature of the water could increase or decrease. It is used to measure heat of combustion. It is used to measure heat of solution or reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The