
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:.
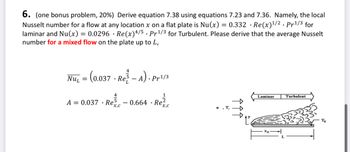
6. (one bonus problem, 20%) Derive equation 7.38 using equations 7.23 and 7.36. Namely, the local
Nusselt number for a flow at any location x on a flat plate is Nu(x) = 0.332 · Re(x) 1/2. Pr 1/3 for
laminar and Nu(x) = 0.0296 Re(x) 4/5. Pr 1/3 for Turbulent. Please derive that the average Nusselt
number for a mixed flow on the plate up to L,
Turbulent
N
Laminar
Re
4
Nu₁ = (0.037 - Re-A). Pr1/3
NUL
4
A = 0.037 Re - 0.664 · Re
.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 1 steps with 3 images

Knowledge Booster
Similar questions
- Question 1: A steady, two-dimensional, incompressible velocity field has cartesian velocity components u = Cy(x² + y²) and v=-Cx(x² + y²), where C is a constant. Transform these cartesian velocity components into cylindrical velocity components u, and ua, simplifying as much as possible. You should recognize this flow. What kind of flow is this? Is this flow follow the continuity equation?arrow_forwardExperiments conducted for a dilute system under laboratory conditions approximating plug flow give HTUOx= 3 ft. If a commercial column is to be designed for NTUOx = 4, with NPex and NPey estimated to be 19 and 50, respectively, determine the necessary column height if E = 0.5.arrow_forwardIn assignment 3 I gave you the following expression for the pressure drop for turbulent flow through a horizontal smooth pipe of constant diameter at the special condition to which the Blasius equations applies: 0.25 L 0.75 1.75 AP = 0.241. BONUS Derive this equation. μ D4.75arrow_forward
- HOMEWORK-2 Considering the steady-state laminar flow of a liquid with density p and viscosity p in a vertical tube of length L and radius R; a) Show the momentum flux and velocity profile in the figure. b) Determine the momentum flux distribution, the velocity distribution, the maximum velocity (Vzmax), and the average velocity (Vzaverage) expressions. PL L R ! Po 1111111 Flow inarrow_forwardMay I ask if a block of copper falling into a tank filled with water is considered a closed or open steady system?arrow_forward2. Two incompressible viscous fluids of the same density p flow, one on top of the other, down an inclined plane making an angle a with the horizontal. Their viscosities are µi and µ2, the lower fluid is of depth hị and the upper fluid is of depth h2. Show that: u,(y) = (hj +h2. 2 (t)- )y - 1 2 gsin a V1 so that the velocity of the lower fluid u¡(y) is dependent on the depth h2, but not the viscosity, of the upper fluid. Why is this?arrow_forward
- A Newtonian fluid with constant density flows in a parallel-plate apparatus that separated by a distance d and length L as shown in Figure 1. The top plate is moving in z-direction with a velocity uw. Derive the velocity distribution of the fluid, vz as a function of y using equation of motion in Appendix 1. List the postulates and you may neglect the gravity force.arrow_forwardCould you explain every step thank youarrow_forwardQuestion in imagearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The