
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The kinetics of this reactant were studied as a function of temperature. (The reaction is first order in each reactant and second order overall.)
C2H5Br(aq) + OH-(aq) Yields C2H5OH(l)+ Br- (aq)
a. Determine the activation energy and frequency factor for the reaction.
b. Determine the rate constant at 15 C.
c. If a reaction mixture is 0.155 M in C2H5Br and 0.250 M in OH- , what is the initialrate of the reaction at 75 C?
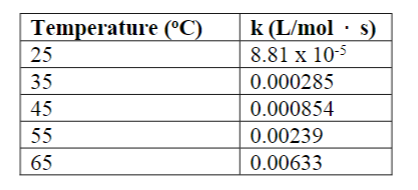
Transcribed Image Text:Temperature (C)
k (L/mol · s)
8.81 x 10-5
0.000285
25
35
45
0.000854
55
0.00239
65
0.00633
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 9 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q 17arrow_forward7. Cyclobutane decomposes to ethene in a first-order reaction. From measurements of the rate constant (k) at various absolute temperatures (T), the accompanying Arrhenius plot was obtained (In k versus 1/T). | a. Calculate the energy of activation, Ea. b. Determine the value of the rate constant at -3 740. K. (In the plot, the units of k are s1.) -4 -5 -6 -7 -8 0.00125 0.0013 0.00135 0.0014 0.00145 1/T (1/K) In karrow_forwardThe rate of the reaction: 2HgCl2 + C2O4-2 → 2Cl- + 2CO2 + Hg2Cl2, is found by measuring the number of moles of Hg2Cl2 that precipitates per liter of solution per minute.arrow_forward
- 10. The rate of a particular reaction increases from 0.10 M s¹ to 0.50 M s¹ when the temperature is increased from 25°C to 35°C. Calculate the activation energy for this reaction.arrow_forward15. What data should be plotted to show that experimental concentration data fits a first-order reaction? A. 1/[reactant] vs. time B. [reactant] vs. time C. ln[reactant] vs. time D. ln(k) vs.1/T E. ln(k) vs. Eaarrow_forward16. What data should be plotted to show that experimental concentration data fits a second-order reaction? A. ln[reactant] vs. time B. [reactant] vs. time C. ln(k) vs. 1/T D. 1/[reactant] vs. time F. ln(k) vs. Eaarrow_forward
- Consider the following reaction: X3 +Y2 --> 3Z2 +H2O the initial reaction rates were measured at 310*C with the following results: a. What is the rate law for this reaction ? b. What is the overall reaction order ? c. What is the rate constant for this reaction ?arrow_forward14. Consider the following reaction: 2 N₂O(g) → 2 N2(g) + O2(g) rate - k[N₂O] For an initial concentration of N₂O of 0.50 M, calculate the concentration of N₂O remaining after 2.0 min ifk -6.8 x 10³ s¹¹. A) B) 0.98 M 0.39 M C) 0.86 M D) 0.22 M E) 0.75 Marrow_forward3. A reaction has a rate constant of 0.000122 s¹ at 27.0°C and 0.228 s'at 77.0°C. a. Determine the activation energy for this reaction. b. What is the value of the rate constant at 17.0°C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY