
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:Temperature (°C)
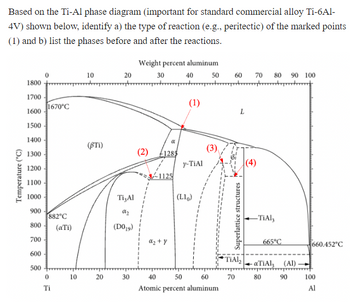
Based on the Ti-Al phase diagram (important for standard commercial alloy Ti-6Al-
4V) shown below, identify a) the type of reaction (e.g., peritectic) of the marked points
(1) and b) list the phases before and after the reactions.
10
20
Weight percent aluminum
30
40
50
60 70 80 90 100
1800
1700
1670°C
(1)
1600
L
1500
1400
a
(ẞTi)
1300
1285
y-TiAl
1200
1100
1125
لسسسلسسسل
1000
Ti, Al
(L10)
900
ር?
882°C
800
(aTi)
(D019)
700
600
500
0
10
20
Ti
Superlattice structures
-TiAl,
665°C
660.452°C
TiAl₂
aTiAl3 (Al).
20
30
40
50
60
70 80
90
100
Atomic percent aluminum
PM
Al
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- QI/ Draw a thermal equilibrium diagram for the binary alloy system (Si –Au), from the following data:- a- Si melts at 1414 °C, and Au melts at 1064 °C , and identify all phases are present in the diagram. b- Eutectic is formed at 360°C containing 20 Wt% Si -80 Wt% Au . c- Determine the amount of each phase for the alloy which consist 60 Wt% Si- 40Wt % Au at 1200 °C and 800 °C ,then determine the amount of eutectic at 200 °C?arrow_forwardConsider the binary Magnesium-Lead phase diagram below.arrow_forwardCan you please solve this and show all of your workarrow_forward
- Can you please solve this and show all of your workarrow_forwardFor the alloy Mg-45 wt% alloy at 500 C, what is the weight percentage of Pb in the alpha phase?arrow_forwardCalculate the weight fraction for phases at point B? what are those phases? Is there any eutectic rection? How many phases are present in this diagram? Cu-Ni system T(°C) TA 1300 -L (liquid): A tie ļine liquidus L +a Тв EB solidus (solid) 1200 TD ID 20 3032 35: 4043 50 Ca wt% Niarrow_forward
- Q1 / Two metals Beryllium (Be) and Silicon (Si) have melting points 1282°C and 1414 °C respectively, are completely soluble as liquids but completely insoluble as solids . They form a eutectic at 1090°C containing 61 wt% Si -39wt% Be? Determine the following:- 1- Draw the thermal equilibrium phase diagram and identify all phases are present in diagram ,then sketches what happens in microstructure when the alloys containing , a- 10wt% Si, b-70wt%Si , solidify completely. 2- Determine the composition and the amount of each phase for the alloy which contain 15 wt% Si-85wt% Be at 1150°C If the alloy is hypoeutectic determine the amount of eutectic at 600 °C? or hypereutectic andarrow_forwardFor the given Ti-Cu phase diagram, specify all temperature-composition points at which eutectics, eutectoids, peritectic, and congruent phase transformations occur. Also, for each, write the reaction upon cooling. Temperature (°C) 1700 1500 1300 1100 900 700 500 0 (Ti) 1 B a 20 Ti₂Cu 40 Composition (wt% Cu) L TiCu TigCu4 60arrow_forwardSolve correctly please.arrow_forward
- iii) For a 68 wt% Zn-32 wt% Cu alloy, make schematic sketches of the microstructure that would be observed for conditions of very slow cooling at the following temperatures: 1000°C, 760°C, 600°C and 400°C. Label all phases and indicate their approximate compositions. Comportion a In 20 40 60 100 1200 |2200 H2000 Liquid 1000 - 1800 デ+ダ H1600 J400 E 1200 600 400 『+キ 600 200 400 40 60 Conpositon tet% Zroarrow_forwardFor 40 wt% Sn – 60 wt% Pb alloy at 150 oC (at point B) as shown in Figure, i. State the name of phase(s) present. ii. Calculate the composition(s) of the phase(s) present. 600 300 Liquid 500 a+L 200 400 300 100 200 100 20 60 100 (Pb) (Sn) Composition (wt % Sn) Temperature ("C) Temperature ("F)arrow_forwardUse the Lead-Tin (Pb-Sn) phase diagram below to answer the following questions: Place the following microstructures in the correct order of formation for slowly cooling a 98 wt% Sn alloy from 300 °C to room temperature (point A to point B).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY