
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please answer

Transcribed Image Text:Task 3. Find all the 10 elements that can be found
on the puzzle. Write the element
number, and its atomic mass. Answers only.
symbol, atomic
WYHJ MSCFO
BNGHNMKWS
CFGTH JKLM
FVBN M OL0
ERLGH
FVEN M
ERAGHNCM K
FVDN MROL
FWYHE MSCF
BNCMNMKWS
CFGTH JKLMDCFG T
FVBN M OL0
ERFG HNJ
FVBNM
ERFGH|T
FWYHJ
OFWY H J
MSCFG
X8NG
NCT
N
KWSX
MID
DCFGTHJKL MD
YFVBN
QERFG
SFVB N
CERFG
FFVBN
GFWY H
ILVER
MAOLOP
HRJMKQ
M GOA OS
HOJGKC
MNONOF
JMSEF G
NMKSSX
HJKI MD
MIOUOP
PFVBN
KQERFGHNJMKQ
VBN
SF
HK ERF0
INEON
HNJMKC
XI0LOF
YSCFG
NMGWS X
OG HJKEMD
F GN OLNP
E HNJMKQ
I0LOS
CERFG NJMKC
M
HO YF
VB N
DNDFWY H
RN H
FVBNP
FWYY J
BNRHNLKWS
CKGTHIKLMD
FVBN M UOL0
ERFG HM
FVBNM IOL0
ERFGHNJMK
FVBNM
MKQE R
SFVB N
LOFFVBNM OOL OF
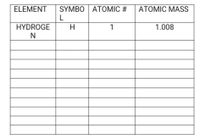
Transcribed Image Text:SYMBO | ATOMIC #
L
ELEMENT
ATOMIC MASS
HYDROGE
N
H
1
1.008
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- rsity o... ingsbo... kboard... ARED4 ethod? 7... F2 Remaining Time: 1 hour, 22 minutes, 23 seconds. Question Completion Status: A Moving to the next question prevents changes to this answer. Question 1 # The angle of the water molecule (H₂O) is 180 degrees 120 degrees 100 degrees 90 degrees 109 degree A Moving to the next question prevents changes to this answer. MAR 14 80 F3 SA $ 000 F4 tv % F5 NA MacBook Air 22 F6 ∞r F7 45.113arrow_forwardInteraction between two protons ?arrow_forwardHO- 1) DMSO, (COCI)2 2) Et,N :? Editarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY