tabletop 91.41m±0.05m cube ruler clipper micrometer Triple beam balai digital balance 3.30cm±0.05cm 2.184±0.001cm 27.310±0.005mm 64.42±0.05g 64.58±0.01g 2.92m±0.05m 2.71cm±0.05cm 2.482±0.001cm 25.251+0.005mm 216.20m±0.05m 2.50cm±0.05cm 3.136±0.001cm 34.140±10.0005mm Part III (Density of Aluminum block) 1. Measure the dimensions of the aluminum block with the metric ruler, vernier calipers, and micrometers 2. Measure the mass with the block with the digital balance and triple-beam balance. 3. Calculate the volume and uncertainty of the block using the dimensions obtained from the metric ruler, vernier calipers, and micrometers. (Calculate the uncertainty o, of the block using the derived equation in Part II.) 4. Using the error propagation equation derive an expression for the uncertainty σ, for the density of the block in terms of the uncertainty of the mass σ and the uncertainty of the volume ov. 5. Calculate the density and uncertainty of the block by using the measurements obtained from the triple-beam balance and metric ruler. 6. Calculate the density and uncertainty of the block by using the measurements obtained from the digital balance and vernier caliper. 7. Calculate the density and uncertainty of the block by using the measurements obtained from the digital balance and micrometer. Data Analysis 1. Calculate the % error between your calculate value of density and the expected value of 2.699 g/cm³. 2. Which of the 3 densities gave the most accurate answer? Was this what you expected why or why not? Explain! 3. Was the propagating error involved in calculating the density significant with any combination of the measuring devices? Explain. 4. What were the random errors involved and how did they affect the density and uncertainty calculation? 5. What systematic errors were involved? 6. Comment on any other sources of error that could have been involved. 5
tabletop 91.41m±0.05m cube ruler clipper micrometer Triple beam balai digital balance 3.30cm±0.05cm 2.184±0.001cm 27.310±0.005mm 64.42±0.05g 64.58±0.01g 2.92m±0.05m 2.71cm±0.05cm 2.482±0.001cm 25.251+0.005mm 216.20m±0.05m 2.50cm±0.05cm 3.136±0.001cm 34.140±10.0005mm Part III (Density of Aluminum block) 1. Measure the dimensions of the aluminum block with the metric ruler, vernier calipers, and micrometers 2. Measure the mass with the block with the digital balance and triple-beam balance. 3. Calculate the volume and uncertainty of the block using the dimensions obtained from the metric ruler, vernier calipers, and micrometers. (Calculate the uncertainty o, of the block using the derived equation in Part II.) 4. Using the error propagation equation derive an expression for the uncertainty σ, for the density of the block in terms of the uncertainty of the mass σ and the uncertainty of the volume ov. 5. Calculate the density and uncertainty of the block by using the measurements obtained from the triple-beam balance and metric ruler. 6. Calculate the density and uncertainty of the block by using the measurements obtained from the digital balance and vernier caliper. 7. Calculate the density and uncertainty of the block by using the measurements obtained from the digital balance and micrometer. Data Analysis 1. Calculate the % error between your calculate value of density and the expected value of 2.699 g/cm³. 2. Which of the 3 densities gave the most accurate answer? Was this what you expected why or why not? Explain! 3. Was the propagating error involved in calculating the density significant with any combination of the measuring devices? Explain. 4. What were the random errors involved and how did they affect the density and uncertainty calculation? 5. What systematic errors were involved? 6. Comment on any other sources of error that could have been involved. 5
Related questions
Question
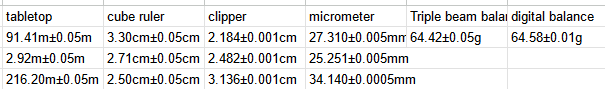
Transcribed Image Text:tabletop
91.41m±0.05m
cube ruler
clipper
micrometer Triple beam balai digital balance
3.30cm±0.05cm 2.184±0.001cm 27.310±0.005mm 64.42±0.05g 64.58±0.01g
2.92m±0.05m 2.71cm±0.05cm 2.482±0.001cm 25.251+0.005mm
216.20m±0.05m 2.50cm±0.05cm 3.136±0.001cm 34.140±10.0005mm

Transcribed Image Text:Part III (Density of Aluminum block)
1. Measure the dimensions of the aluminum block with the metric ruler, vernier
calipers, and micrometers
2. Measure the mass with the block with the digital balance and triple-beam balance.
3. Calculate the volume and uncertainty of the block using the dimensions obtained
from the metric ruler, vernier calipers, and micrometers. (Calculate the uncertainty
o, of the block using the derived equation in Part II.)
4. Using the error propagation equation derive an expression for the uncertainty σ, for
the density of the block in terms of the uncertainty of the mass σ and the
uncertainty of the volume ov.
5. Calculate the density and uncertainty of the block by using the measurements
obtained from the triple-beam balance and metric ruler.
6. Calculate the density and uncertainty of the block by using the measurements
obtained from the digital balance and vernier caliper.
7. Calculate the density and uncertainty of the block by using the measurements
obtained from the digital balance and micrometer.
Data Analysis
1. Calculate the % error between your calculate value of density and the expected value
of 2.699 g/cm³.
2. Which of the 3 densities gave the most accurate answer? Was this what you expected
why or why not? Explain!
3. Was the propagating error involved in calculating the density significant with any
combination of the measuring devices? Explain.
4. What were the random errors involved and how did they affect the density and
uncertainty calculation?
5. What systematic errors were involved?
6. Comment on any other sources of error that could have been involved.
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
