
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
How do I calculate, Table 4.
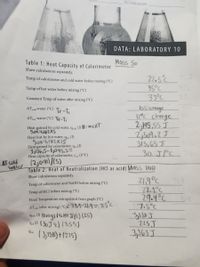
Transcribed Image Text:SECTION/GROUP:
DATA: LABORATORY 10
Table 1: Heat Capacity of Calorimeter
Mass 50
Show calculations separately.
22.3
22.5°c
45°C
33°C
l0:5 change
I2°C change
2,195.55 J
2,501.2J
313,65 J
30.J1°C
Temp of calorimeter and cold water before mixing (°C)
Temp of hot water before mixing (°C)
35
Constant Temp of water after mixing (°C)
AT cold Water (°C)
TF-Ti
AT hot Water (°C) Tf-Ti
Heat gained by cold water, q.l (J) H= MCAT
5ox 4.162X5
Heat lost by hot water, qhot (J)
Sox i,192X15
Heat gained by calorimeter, q (J)
3,136,5-1,045.5=
Heat capacity of calorimeter, c (J/°C)
HAT Cold
water
12,091)/(5)
Table 2: Heat of Neutralization (HCI as acid) Mass loo
Show calculations separately.
219°C
72.1°C
29.4°C
317.5°C
3y138 J
225J
3,363J
Temp of calorimeter and NaOH before mixing (°C)
Temp of HCl before mixing (°C)
Final Temperature extrapolated from graph (°C)
AT , (after mixing) ("C29.4-21.9=7.5°c
9ad () (lo69) (4.164 Jlge) (7.5)
Su0 (30 J %) (7,5°)
- (3,138) + (215)

Transcribed Image Text:Table 3: Heat of Neutralization (HC,H,0, as acid) Nd55 100
Show calculations separately.
22.3°C
Temp of calorimeter and NaOH before mixing (°C)
Temp of HC,H,O, before mixing (°C)
Final Temperature extrapolated from graph (°C)
28.5°C
28.5
AT,oln (after mixing) (*C) 28,5-22,3
6,2°C
१. () (\oog) (५,84) (.2)
9ai (1) [30J) (6)
2594J
184 J
2)780
A (2, squ) + 1186)
Table 4:Reactions and Reac tion Stoichiometry
Show calculations separately.
HCl + NaOH→
НС Н,О, + NaОН —
moles of water from the reaction with HCI
AHHC Rxn (kJ/mol)
moles of water from the reaction with HC,H,O,
AH HC,H,0, Rxn (kJ/mol)
Calculations
(For each calculation step above, give one example of how the calculation was completed and the
equation used.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If a sample weighing 51.0 g contains only carbon and hydrogen, and contains 14.0 g of hydrogen, what is the percentage of hydrogen by mass in the sample? Please enter the value only with one decimal placearrow_forwardItem 10 10 of 33 Complete I Review | Constants I Periodic Table Part A The compound MgCl2 is named dimagnesium chloride. magnesium chlorine. magnesium (II) chloride. magnesium dichloride. magnesium chloride.arrow_forwardWhat is the percent by mass NaCl of a solution that contains 85.7 grams of NaCl and 104.1 grams of water? Round your answer to 1 decimal.arrow_forward
- Please don't provide hsndwritten solution .....arrow_forwardIf a water sample has 0.07 mol of calcium ions in 15 L solution, what is the water hardness level in mg/L? Pay attention to the unit. Hint: For the calcium mass , convert mol to g to mg. (1 mol calcium ion= 40.08 g/mol and 1 g = 1000 mg) Round and report your answer to an integer without decimal place. Report numeric value only, no unit. • Water hardness levels according to EPA Soft: 0-60 milligrams per liter (mg/L) as calcium carbonate Moderately hard: 61-120 mg/L as calcium carbonate • Hard: 121-180 mg/L as calcium carbonate Very hard: more than 180 mg/L as calcium carbonatearrow_forwardWhat is -30°F in K? O 30K ЗОК O 303 K О-30 К 0239 К 04Karrow_forward
- The chemical formula for cesium chloride is CsCl . A chemist measured the amount of cesium chloride produced during an experiment. She finds that 9.25 g of cesium chloride is produced. Calculate the number of moles of cesium chloride produced. Round your answer to 3 significant digits.arrow_forwardOne of the Ionic compounds in sports drinks is potassium di hydrogen phosphate. The label on one of these sports drinks tells us that 240 mL of the solution contains 30 mg of potassium. KH2PO4 is the only ingredient that can provide potassium. how many grams of KH2PO4 are dissolved in 240 mL of the solution? What is the molars ty of KH2PO4 in this solution? Organize abs label your calculations so that they are presented in a logical manner for full creditarrow_forwardMagnesium will burn in air to form both Mg3N2 and MgO. What mass of each product would be found if burning a 3.29 g sample of magnesium to completion produces a combined total of 5.09 g of the two products?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY