
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
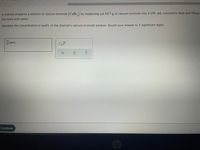
Transcribed Image Text:A chemist prepares a solution of calcium bromide (CaBr,) by measuring out 84.7 g of calcium bromide into a 150. mL volumetric flask and filling
the mark with water.
Calculate the concentration in mol/L of the chemist's calcium bromide solution. Round your answer to 3 significant digits.
| molL
Continue
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please see attached imagearrow_forwardA chemist prepares a solution of barium chloride (BaCl,) by measuring out 17.1 g of barium chloride into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's barium chloride solution. Round your answer to 3 significant digits. I molLarrow_forwardA chemist prepares a solution of sodium nitrate (NaNO,) by measuring out 83.1 g of sodium nitrate into a 100. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's sodium nitrate solution. Round your answer to 3 significant digits. mol/L x10 ?arrow_forward
- Please don't provide handwritten solution ...arrow_forwardA chemist prepares a solution of nickel (II) chloride (NiCl2) by measuring out 160. umol of nickel (II) chloride into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's nickel (II) chloride solution. Round your answer to 3 significant digits.arrow_forwardSuppose 0.377 g of potassium chloride is dissolved in 100. mL of a 37.0 m M aqueous solution of silver nitrate. Calculate the final molarity of chloride anion in the solution. You can assume the volume of the solution doesn't change when the potassium chloride is dissolved in it. Be sure your answer has the correct number of significant digits. M D x10 X Sarrow_forward
- A chemist prepares a solution of potassium iodide KI by measuring out 88.6g of potassium iodide into a 100.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's potassium iodide solution. Round your answer to 3 significant digits.arrow_forwardA chemist must dilute 42.4 mL of 483. mM aqueous potassium dichromate (K,Cr,O,) solution until the concentration falls to 380. mM . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits. mL alaarrow_forwardA chemist prepares a solution of copper(II) sulfate (CuSO,) by measuring out 12. g of copper(II) sulfate into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's copper(II) sulfate solution. Round your answer to 2 significant digits. mol/Larrow_forward
- How many moles of sodium hydroxide, NaOH, are present in 1.73 L of a 0.157 M sodium hydroxide solution? Place your answer in the box. Express your answer using only a number without text. Report your result in decimal notation and to the proper number of significant figures. All numbers are measured.arrow_forwardPlease see imagearrow_forwardA chemist prepares a solution of iron(II) bromide (FeBr,) by measuring out 63. g of iron(II) bromide into a 150. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's iron(II) bromide solution. Round your answer to 2 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY