
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Tartaric acid is found in many fruits, including grapes, and is
partially responsible for the dry texture of certain wines. Calculate
the pH and the tartrate ion (C4H4O62-) concentration
for a 0.250 M solution of tartaric acid, for which the acid-dissociation
constants are listed in Table . Did you have to make
any approximations or assumptions in your calculation?
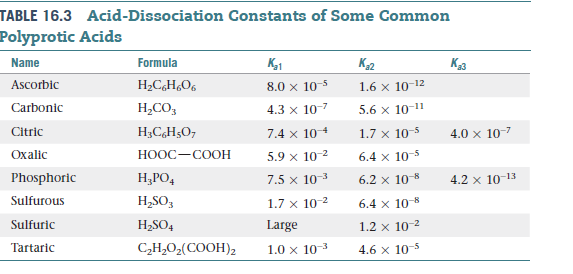
Transcribed Image Text:TABLE 16.3 Acid-Dissociation Constants of Some Common
Polyprotic Acids
Name
Formula
K1
K2
Ascorbic
H2CH¿O6
8.0 x 10-5
1.6 x 10-12
Carbonic
Н.СО,
4.3 x 10-7
5.6 x 10-1"
Citric
H;C,H5O7
7.4 x 104
1.7 x 10-5
4.0 x 107
Охalic
HOOC-COOH
5.9 x 10-2
6.4 x 10-5
Phosphoric
Н,РО,
7.5 x 10-3
6.2 x 10-8
4.2 x 10-13
Sulfurous
H,SO,
1.7 x 10 2
6.4 x 108
Sulfuric
H2SO4
Large
1.2 x 10-2
Tartaric
С-Н.О,(СООН)2
1.0 x 10-
4.6 x 10-5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part A Reset Help significant When determining the pH of a polyprotic acid solution or mixture of acids, only the [OH | acid needs to be considered. Any another other ionizations are by comparison, and ion content. the overall negligible must be considered in does not contribute to strongest weakest [H;O]+arrow_forwardA solution of a weak acid is 0.20 M and is 0.76 % ionized. Determine Ka.arrow_forwardIf the molar concentration of OH- is 1.0 x 10-5, the pH is (answer) and the solution is (answer), acidic, basic, neutral?arrow_forward
- Give detailed Solution....don't give Handwritten answer..don't use Ai for answering thisarrow_forwardA 0.250M solution of an unknown acid has a pH of 5.473. What is the Ka Answer: of this acid?arrow_forwardwrite the reacrion for the acidic and basic Salts mohen It is dissolied M waier. Be Sure Yo write The met ionic eq. and elomiote specreors ions and elimiore That are neutral, clou/ Cotg cooNo, KclO4 .cooNaarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY