
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:16 of 21
I Review | Constants | Periodic Ta
Part B
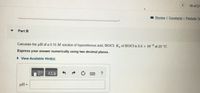
Calculate the pH of a 0.10 M solution of hypochlorous acid, HOCI. K. of HOCI is 3.5 x 10 * at 25 °C.
-8
Express your answer numerically using two decimal places.
• View Available Hint(s)
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 °C. conjugate acid formula conjugate base Ка formula Ко ✗ HSO 0.012 ☐ ☐ -4 CH3NH2 4.4 × 10 ☐ CHN 1.7 x 10 -9 ☐ x10arrow_forwardSolutions lons pH meter Water + 7.00 но", он 3 Strong acid А , н + 2.00 Weak acid + 4.50 A , H 0 3 Strong base M *, + OH 12 Weak base 9.50 ВН OH Formula: pH = - log[H*] a. Using the formula given, calculate the hydronium ion concentration of all the solutions in Table 1. Comment higher and explain which solution has a concentration value why?arrow_forwardCalculate the pH and pOH of the following solutions :1- 0.01 M HBr2- 0.00005 M HBr3- 0.00002 M KOH4- 0.01 M CH COOH5- 0.00001 M CH,COOH6- 0.01 M HIO,7-0.01 M pyridinearrow_forward
- #8 - thumbs up if you do it correctly! Be sure your answer has the correct number of significant digits as it states in the directions.arrow_forwardThe pH solution ranges from 0 to 14. An acid has a pH less than 7. Pure water is neutral and has a pH of 7. The pH of a solution is given by pH=-logx where x represents the concentration of the hydrogen ions in the solution in moles per liter. Find the pH if the hydrogen ion concentration is 2.8x10-4.arrow_forwardFor each of the following entities • Identify it as a strong or weak acid. • Select the correct arrow to show the changes that occur when the acids are placed in water. • Select the approximate pH for each resulting solution. HCI(aq) is classified as a + acid. The reaction equation with water utilizes which of the following arrows? Choice A Choice B 7 >>7 H2SO3(aq) is classified as a The reaction equation with water would utilize which of the following arrows? Choice A Choice B < 50% H2SO3(aq) + H20(I) • HSO3 (aq) + H30*(aq) The approximate pH of the solution would bearrow_forward
- Be sure to answer all parts. Please report your answer to the correct number of significant figures. Determine the K, of a weak base if a 0.617 M aqueous solution of the base at 25°C has a pH of 10.88. x 10 (Enter your answer in scientific notation.)arrow_forwardhelp pleasearrow_forward-8 Calculate the pH of a 4.9 x 10 M aqueous solution of hydroiodic acid (HI). Round your answer to 2 decimal places.arrow_forward
- Nicotinic acid, HC,H&NO2, is a B vitamin. It is also a weak acid with K, = 1.4 x 10-5. Calculate [H+] and the pH of a 0.034 M solution of HCGH4NO,. [H*] = Use correct number of significant digits; pH = Use correct number of significant digits;arrow_forwardi need help solving part a and b of this question pleasearrow_forwardInitial pH: 10.18 Kb: 5.13x10^-5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY