
College Physics
10th Edition
ISBN: 9781285737027
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A sauna has 8.5 m3 of air volume, and the temperature is
85°C. The air is perfectly dry. How much water (in kg)
should be evaporated if we want to increase the relative
humidity from 0% to 10%? (See Table 13–3.)
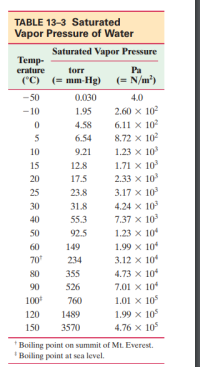
Transcribed Image Text:TABLE 13-3 Saturated
Vapor Pressure of Water
Saturated Vapor Pressure
Тemp-
erature
(°C) (= mm-Hg) (= N/m³)
torr
Ра
- 50
0.030
4.0
- 10
1.95
2.60 x 102
6.11 х 10?
8.72 x 102
4.58
6.54
10
9.21
1.23 x 103
1.71 × 103
2.33 x 103
15
12.8
20
17.5
25
23.8
3.17 х 103
4.24 x 103
7.37 x 103
30
31.8
40
55.3
50
92.5
1.23 x 104
60
149
1.99 х 104
70*
234
3.12 х 104
80
355
4.73 x 104
90
526
7.01 x 104
100*
760
1.01 × 105
1.99 × 105
4.76 × 105
120
1489
150
3570
Boiling point on summit of Mt. Everest.
* Boiling point at sea level.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The data in the following table represent measurements of the masses and dimensions of solid cylinders of aluminum, copper, brass, tin, and iron. (a) Use these data to calculate the densities of these substances. (b) State how your results compare with those given in Table 14.1.arrow_forwardYou are looking to purchase a small piece of land in Hong Kong. The price is “only” 60,000 per square meter! The land title says the dimensions are 20m30m. By how much would the total price change if you measured the parcel with a steel tape measure on a day when the temperature was 20C above normal?arrow_forwardHow many cubic meters of helium are required to lift a light balloon with a 400-kg payload to a height of 8 000 m? Take Hc = 0.179 kg/m3. Assume the balloon maintains a constant volume and the density of air decreases with the altitude z according to the expression pair = 0e-z/8 000, where z is in meters and 0 = 1.20 kg/m3 is the density of air at sea level.arrow_forward
- (a) Suppose a meter Stick made of steel and one made of invar (an alloy of iron and nickel) are the same length at 0C. What is their difference in length at 22.0C ? (b) Repeat the calculation for two 30.0mlong surveyor's tapes.arrow_forwardIn the ideal gas law, pressure is ___ proportional to volume. (5.6)arrow_forwardReview. While you arc on a trip to Europe, you must purchase hazelnut chocolate bars for your grandmother. Eating just one square each day, she makes each large bar last for one and one-third months. Mow many bars will constitute a years supply for her?arrow_forward
- . On a winter day, the air temperature is — 15°C, and the humidity is 0,001 kg/m3. (a) What is the relative humidity? (b) When this air is brought inside a building, it is heated to 20°C. If the humidity isn't changed, what is the relative humidity inside the building?arrow_forwardGive an example in which density is used to identify the substance composing an object. Would information in addition to average density be needed to identify the substances in an object composed of more than one material?arrow_forwardWhich of the assumptions below is not made in the kinetic theory of gases? (a) The number of molecules is very large. (b) The molecules obey Newtons laws of motion. (c) The forces between molecules are long range. (d) The gas is a pure substance. (e) The average separation between molecules is large compared to their dimensions. (f) of (his account are correct statements necessary for a clear and complete explanation? (ii) Which are correct statements that are not necessary to account for the higher thermometer reading? (iii) Which are incorrect statements?arrow_forward
- Dry air is primarily composed of nitrogen. In a classroom demonstration, a physics instructor pours 2.00 L of liquid nitrogen into a beaker. After the nitrogen evaporates, how much volume does it occupy if its density is equal to that of the dry air at sea level? Liquid nitrogen has a density of 808 kg/m3.arrow_forwardA deepsea diver should breathe a gas mixture that has the same oxygen partial pressure as at sea level, where dry air contains 20.9% oxygen and has a total pressure of 1.01105N/m2. (a) What is me partial pressure of oxygen at sea level? (b) If the diver breathes a gas mixture at a pressure of 2.00106N/m2, what percent oxygen should it be to have the same oxygen partial pressure as at sea level?arrow_forwardThe density of air is 1.3 kg/m3 at sea level. From your knowledge of air pressure at ground level, estimate the height of the atmosphere. As a simplifying assumption, take the atmosphere to be of uniform density up to some height, after which the density rapidly falls to zero. (In reality, the density of the atmosphere decreases as we go up.) (This question is courtesy or Edward F. Redish. For more questions of this type, see http://www.physics.umd.edu/perg/.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Expert Answers to Latest Homework Questions
Q: Lorie Nursery plans to sell 320 potted plants during April and 240 units in May. Lorie Nursery keeps…
Q: general account expert solve.
Q: Assets totaled $25,550 and liabilities totaled $8,630 at the beginning of the year. During the year,…
Q: Compute the cost of each machine. Sub - General Accounting.
Q: Quick answer of this accounting questions
Q: During periods of rising prices which of the following is true:(Accounting)
Q: What was its total assets turnover ratio?
Q: I want to correct answer accounting questions
Q: net income? Cost Account
Q: Baltimore Company experienced an increase in total assets of $10,500 during
the current year. During…
Q: Provide general account answer
Q: What is the equity at the end of the year? for this general account questions.
Q: Do fast answer of this accounting questions
Q: B
B
Canning Machine
2
Monster Beverage is considering purchasing a new canning machine.
This machine…
Q: General Account
Q: Andrews Corporation uses a process costing system for manufacturing.
The following information is…
Q: Provide correct answer general Accounting question
Q: Prove it
Q: Finances
Income Statement
Balance Sheet
Finances
Income Statement
Balance Sheet
Materia
Income…
Q: SA
[(a) 5 V (b) 5 V]
13. Find the voltage V in the network shown in Fig. 2.44 (a) if R is 10 2 and…
Q: O Draw the four possible negative feedback
contigurations of an op-amp. Write the
input and output…