
Nitrogen injection to recover natural gas.
Nitrogen is injected into oil wells to increase the recovery of crude oil (enhanced oil recovery). It mixes with the natural gas that is produced along with the oil. The nitrogen must then be separated from the natural gas. A total of 170,000 SCFH (based on 60oF and 14.7 psia) of natural gas containing 18% N2, 75% CH4, and 7% C2H6 at 100oF and 800 psia is to be processed so that it contains less than 3 mol% nitrogen in a two-step process:
(1) membrane separation with a nonporous glassy polyimide membrane, followed by (2) pressure-swing adsorption using molecular sieves highly selective for N2( SPN2;CH4= 16) and completely impermeable to ethane. The pressure-swing adsorption step selectively adsorbs methane, giving 97% pure methane in the adsorbate, with an 85% recovery of CH4 fed to the adsorber. The non-permeate (retentate) gas from the membrane step and adsorbate from the pressure-swing adsorption step are combined to give a methane stream that contains 3.0 mol% N2. The pressure drop
across the membrane is 760 psi. The permeate at 20oF is compressed
to 275 psia and cooled to 100o F before entering the adsorption step. The adsorbate, which exits the adsorber during regeneration at 100oF and 15 psia, is compressed to 800 psia and cooled to 100oF before being combined with non-permeate gas to give the final pipeline natural gas.
(a) Draw a flow diagram of the process using appropriate symbols.Include compressors and heat exchangers. Label the diagram with the data given and number all streams.
(b) Compute component flow rates of N2, CH4, and C2H6 in lbmol/h
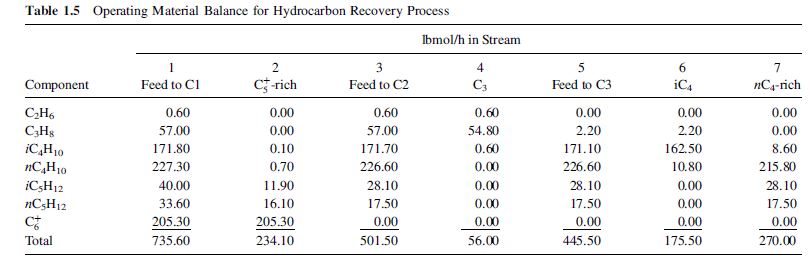
in all streams and create a material-balance table similar to Table 1.5.
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

- Using a psychrometric diagram, depict the heating and humidification process from the following data, Initially the air is at dry bulb temperature (DBT) 40 ° C and RH 50%. The air is heated to DBT 90 ° C. The heated air is then flowed through a humidifier to increase the RH to 25%. For this process, calculate a Change in the absolute humidity of the air from initial to final conditions = ... (kg of water / kg of air) b. The change in air enthalpy from initial to final conditions = ... (kJ / kg air)arrow_forwardTwo aqueous NaOH streams are combined. Stream A is 26% NaOH by mole. Stream B is 69% NaOH by mole. The product (combined) stream has a flow rate of 125 lbmole/h and is 57% NaOH by mole. Determine the flow rate of stream A and stream B (lbmole/h).arrow_forwardQ.1) In the experiment, you wrap a piece of copper wire around your magnesium strip to suspend the magnesium inside the eudiometer. Why is copper wire a good choice for this task? Group of answer choices It conducts electricity very well It reacts strongly with the acid It does not react with the acid It is much heavier than Mg Part B) A 45 mL sample of a dry gas is collected at 380 torr and 25 °C. Calculate the volume of the gas sample (in mL) at STP. Part C)Using the data provided in Table 3 in the handout (also provided below), calculate the vapor pressure of water at 21.0 °C. Table 3: Vapor pressure of water at various temperatures T (˚C) P (mmHg) T (˚C) P (mmHg) T (˚C) P (mmHg) 0 4.58 16 13.63 26 25.21 5 6.54 18 15.48 28 28.35 10 9.21 20 17.54 30 31.82 12 10.52 22 19.83 40 55.3 14 11.99 24 22.38 50 92.5arrow_forward
- A student uses a polystyrene calorimeter to determine the enthalpy of reaction of hydrobromic acid with potassium hydroxide. The student mixes 100.0 mL of 0.50 mol/L HBr(aq) at 21.0oC with 100.0 mL of 0.50 mol/L KOH(aq), also at 21.0oC. The highest temperature reached is 24.4oC. Write the thermochemical equation of the reaction.arrow_forwardUsing a psychrometric diagram, depict the heating and humidification process from the following data, Initially the air is at dry bulb temperature (DBT) 20 ° C and RH 35%. The air is heated to DBT 70 ° C. The heated air is then flowed through a humidifier to increase the RH to 25%. For this process, calculate a Change in the absolute humidity of the air from initial to final conditions = ... (kg of water / kg of air) b. The change in air enthalpy from initial to final conditions = ... (kJ / kg air)arrow_forwardA liquid mixture containing 30.0 mol% benzene (B), 25.0% toluene (T), and the rest xylene (X) feeds a distillation column. The bottom product contains 98.0 mol% X and no B, and 96.0% of the X in the feed is recovered in this stream. The top product feeds a second column. The top product of the second column contains 97.0% of the B in the feed to this column. The composition of this stream is 94.0 mol% B and the rest T. (a) Draw and label a flow diagram for this process and perform a degree of freedom analysis to prove that, for an assumed basis of calculation, the molar flow rates and compositions of all process streams can be calculated with the given information. Write the equations you would solve in order to calculate the unknown process variables. In each equation (or set of simultaneous equations), indicate the variables you would solve for. Do not perform any calculations yet. (b) Calculate (i) the percentage of benzene in the process feed (i.e., the feed to the first column)…arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





