
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
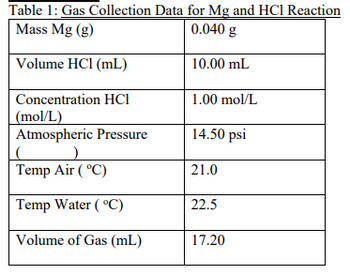
Transcribed Image Text:Table 1: Gas Collection Data for Mg and HCI Reaction
Mass Mg (g)
0.040 g
Volume HC1 (mL)
10.00 mL
Concentration HC1
1.00 mol/L
(mol/L)
Atmospheric Pressure
14.50 psi
C
Temp Air (°C)
21.0
Temp Water (°C)
22.5
Volume of Gas (mL)
17.20

Transcribed Image Text:8. Calculate the % yield of the gas produced in this reaction. Don't forget to state the formula.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Constants Periodic Table Consider the following reaction: If the initial volume of the H2O2 solution is 3.2 L, what total amount of O2 (in moles) is formed in the first 20 s of reaction? Hint: Use Figure 1 to answer this problem. H2O2(aq) H20() + ½ O2(g) and the kinetics plots below to answer the questions to the right. Express your answer in moles to two significant figures. O 1.4 mol Figure 1. Plot of [H,O,] vs time O .8 mol 0.9 0.63 mol 0.8 0.7 O 0.70 mol 0.6 0.5 0.4 Submit Request Answer 0.3 0.2 0.1 Part B 20 40 60 80 time (s) concentration of H;O2 (mol L²)arrow_forwardConsider the neutralization reaction 2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq)2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq) A 0.110 L0.110 L sample of an unknown HNO3HNO3 solution required 54.5 mL54.5 mL of 0.250 M Ba(OH)20.250 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3HNO3 solution?arrow_forwardPls help ASAP. Pls circle the final answer.arrow_forward
- HQ12.22 X Answered. 1 attempt left Find the volume of HCI gas at STP required to prepare 3.50 L of a 0.100 m HCI solution (assume water density = 1.00 g/mL). Numeric answer 8.68 X X Answered - Incorrect Donearrow_forwardest Ort a gamb rement: 56°F Cloudy Sodium chloride (NaCl) has a solubility of 36.0 g/100 g H₂O at 20 °C. Select the temperature at which the solubility of NaCl will be higher than 36.0 g/100 g H₂O among the following. U 20 °C O 10 °C O 40 °C chemistry and reaction of Employ... paymen... O 5 °C receipt % 10 f6 & hparrow_forwardWhat is the concentration of helium dissolved in 3.5 L of water when enclosed in a sealed container and pressurized with 15 atm of helium gas? (kH(He) = 3.7 x 104 mol L-1 atm1) 0.019 mol/L O 0.0016 mol/L 0.056 mol/L O 0.0031 mol/Larrow_forward
- Please help I'm not sure how to solve these. A solution is prepared by dissolving 20g of NaCl (f.w.= 58.44 g mol^-1), a nonvolatile solute, in enough water (m.w. = 18.02 g mol^-1) to result in exactly 1 L of solution at 25 C. The density of the solution is 1 g mL ^-1. How do you calculate freezing point of the solution as well as vapor pressure and the osmotic pressure? I understand boiling point.arrow_forwardg The solubility of Ni(OH)2 in water at 25 °C is measured to be 4.9 × 10-4 Use this information to calculate Ksp for Ni(OH)2. Round your answer to 2 significant digits. 8 0 x10 L 3arrow_forwardYou are asked to prepare a 1.000 L solution of 4.5 M you commit a user error while preparing this solution. Assumed volume Volumetric error Preparation details Added water 2.0 cm above the line, which corresponds to 8.2 mL (0.0082 L) additional solution volume C6H12O6 (glucose; molar mass = 180.16 g/mol) in a lab by dissolving 811.0 g of glucose in water. Consider the following two scenarios in whic Prepared in a beaker Prepared in a volumetric flask 1.000 L You add the glucose to a volumetric flask and then add water until it dissolves. The water bottle you are using has a worn tip, and you inadvertently add too much water such that the meniscus is above the line. The diameter of the neck of the volumetric flask is 2.29 cm. 811.0 g glucose Concentration: You decide to evaluate and compare the errors you made while preparing the solutions using the different methods. Calculate the actual concentrations of the intended 4.5 M glucose solutions prepared by each method based on their…arrow_forward
- Consider the neutralization reaction 2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq)2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq) A 0.125 L0.125 L sample of an unknown HNO3HNO3 solution required 54.3 mL54.3 mL of 0.200 M Ba(OH)20.200 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3HNO3 solution?arrow_forwardWhere stated, classify reactions both by the key reaction event (PPT, A/B, R/O) and by the changes in composition (COM, DEC, SDP, DDP). 21. 2NI3(s) → N2(g) + 31,(g) classify: When 730 mL at STP of N2 form, mL at STP of I2 form as well. To make 55 liters at STP of I2t mol of NI3 must react.arrow_forwardClaim: My answer to the question is.... Evidence: The evidence from the document that supports my answer is... Reasoning: The reasonI chose this evidence is...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY