
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
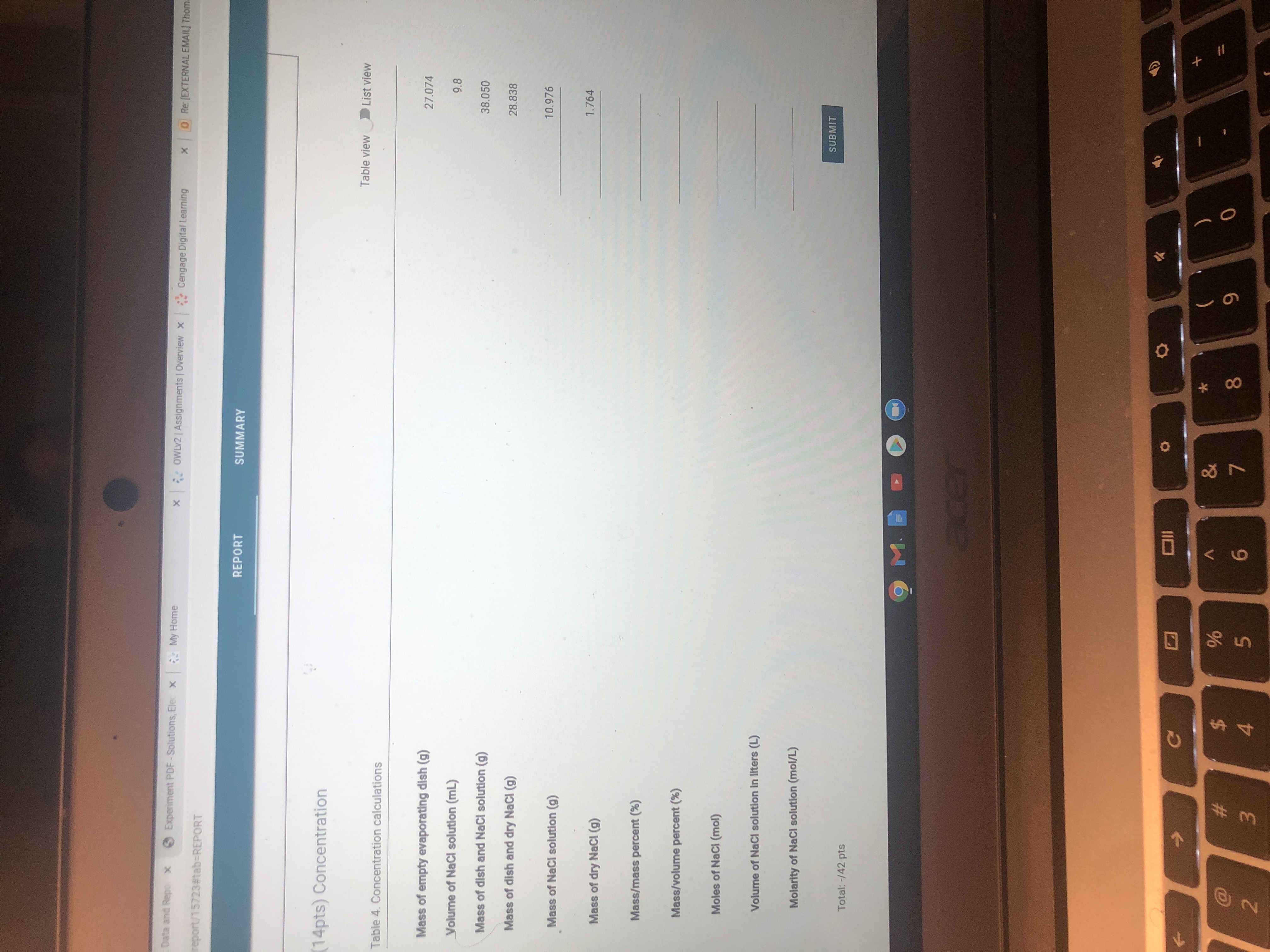
Transcribed Image Text:(14pts) Concentration
Table view
List view
Table 4. Concentration calculations
Mass of empty evaporating dish (g)
27.074
Volume of NaCl solution (mL)
9.8
Mass of dish and NaCl solution (g)
38.050
Mass of dish and dry NaCl (g)
28.838
Mass of NaCl solution (g)
10.976
1.764
Mass of dry NaCl (g)
Mass/mass percent (%)
Mass/volume percent (%)
Moles of NaCl (mol)
Volume of NaCl solution in liters (L)
Molarity of NaCI solution (mol/L)
SUBMIT
Total: -/42 pts
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a solution composition of 10.0 g solute and 90.0 g solvent; choose the correct numerical concentration of % (w/w).arrow_forwardOMacmillan Learning Express the concentration of a 0.0160 M aqueous solution of fluoride, F, in mass percentage and in parts per million (ppm). Assume the density of the solution is 1.00 g/mL.. mass percentage: ppm: 30.4 Incorrect 0.0304 28 ppmarrow_forwardIdentify as true (T) or false (F): ___ Polar solvents tend to attract and dissolve nonpolar solutes. ___ True solutions form only in a liquid state. ___ Solubility of a gas in a liquid depends on the pressure of the gas above the solution. ___ Colligative properties of a solvent depend on the nature of the solute dissolved in it.arrow_forward
- Identify the solute and solvent in each of the solutions. A. table sugar (C12H22O11)(C12H22O11) in water table sugar: water: B. air (a solution of 78% N2,78% N2, 21% O2,21% O2, and various other gases) N2:N2: O2:O2: C. a solution of 30%30% ethanol and 70%70% water ethanol: water: D. bronze (an alloy of 95%95% copper and 5%5% tin) copper: tin: E. CO2(g)CO2(g) in water CO2:CO2: water:arrow_forwardCalculate the final concentration of each solution show calculationsarrow_forwardy Song - CHEM-1405 63... pter 9 Assignment 3 Dok nt nt nces Solubility Song - YouTube % KF ezto.mheducation.com G carbonates examples - Google Search Saved M Question Determine the mass percent composition of a solution made by dissolving 21.1 g of KF in 415 g of H₂O.arrow_forward
- III. Complete the table below. Show complete solutions. Box/Encircde your final answers reported in proper units and number of significant figures. Assume that all solutions were prepared at 25°C, and the solutes were completely dissolved. Molar masses should be based on the CHEM 18.1 Periodic Table provided in Moodle. Density values, if needed, can be found at the last page of this drill. SOLUTION DESCRIPTION MOLARITY (M) MOLALITY (m) %(w/w) 12.58 grams potassium hydroxide (KOH) dissolved in 100.00 ml ethanol Ethanolic КОН ABOGA (assume that the amount of solute has no effect to the volume of the solution)arrow_forwardTrue or false: The mass of K2Cr2O7 (molar mass= 294.19 g/mol-1) needed to make 235 mL of a 0.170 M solution, is 117 g. A) true B) falsearrow_forwardPlease don't provide handwritten solution ....arrow_forward
- Please answer number 7, letters d and e only. Thank youarrow_forward2 students make solutions of CuCl2.2H2O using 25-mL volumetric flask. Student A weights out 2.489g of solid, and student B weight out 1.867g. a.) Briefly give the steps they follow to make the solid into a solution using the 25-mL volumetric flask.arrow_forward101 Chem101 O A useful guide for studying : C x * Chem101 101 Chem101 tps://app.101edu.co Question 8 of 12 What is the concentration in molarity of an aqueous solution which contains 1.69% by mass acetone (MM = 58.08 g/mol)? The density of the solution is 0.971 g/mL. %3Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY