
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Help me to figure out the volume of this equation. Thank you.
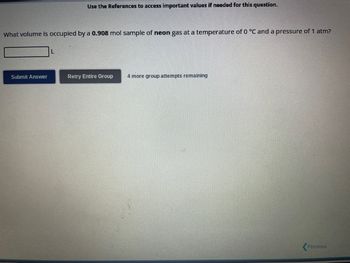
Transcribed Image Text:What volume is occupied by a 0.908 mol sample of neon gas at a temperature of 0 °C and a pressure of 1 atm?
Submit Answer
Use the References to access important values if needed for this question.
L
Retry Entire Group 4 more group attempts remaining
Previous
20X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. For his second experiment, Samuel uses vinegar instead of acetic acid powder. He sets up three experimental trials with different reaction conditions. He records all his data in the table below for the three trials. Trial Mass of Baking Soda (g) Temperature (°C) AVolume of Vinegar (mL) Reaction time to completion (s) 1 10.0 25.0 60.0 41 2 10.0 40.0 60.0 23 3 10.0 55.0 60.0 12 a) Describe a pattern you observe in Samuel's experimental results. In your answer, cite specific data from the table above. BIYE E m Σ 0/ 10000 Word Limit Aa 2048 26 C37.006 tv MacBook Air B88 F10 FS F4 & 23 %3D 4 5 6 8 2 3 { T Y Q W E G H J K A S D M V command option command . .. R Narrow_forwardA mixture of C2H2 and CH4 has a total mass of 164 g. When this mixture reacts completely with excess oxygen, the CO2 and H2O products have a combined mass of 746.6 g. What mass of CH4 was present in the initial mixture?arrow_forwardFor the reaction C + 2H2 → CH4, how many grams of hydrogen are required to produce 9 moles of methane, CH4 ?arrow_forward
- diagram b is correct, What procedures can be used to solve this problem? write down the plan for this new way to solve the problem. 1. first execute plan 1 to solve this problem. explain in every step. 2. solve the problem again - this time use the plan that you propose, plan 2. 3. finally, think about the way that you can use it to make sure that your answer makes sense. write your assessment.arrow_forwardNot too sure if the answer is 124 g or 0.124 g.arrow_forward[Tutorial: Limiting reactant stoichiometry] This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. b) Step 2a: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 64.7 grams Al according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) c) Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 201 grams Fe₂O₃ according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) d) Which of the following substances is the limiting reactant? e) What is the mass in grams of the excess Fe₂O₃ remaining after the partial reaction of 201 g Fe₂O₃ with 64.7 g Al? Give your answer to three significant figures.arrow_forward
- how did you get the numbers beside the balanced equations. such as 4 beside H2O and 2 beside N2.arrow_forwardChemistry GAM The diagram (Figure 1) represents a high-temperature reaction between CH4 and H₂O. Figure Part A Based on this reaction, find how many moles of CO can be obtained starting with 5 mol CH₂? Express your answer as an integer. | ΑΣΦ 1 → C ? n = mol Part B Based on this reaction, how many moles of H₂ can be obtained starting with 5 mol CH4? Express your answer as an integer. IVE ΑΣΦ ? n= Submit Request Answer molarrow_forwardConsider the reaction 3X + 2Y → 5C + 4D With excess Y, how many moles of X are needed to produce 13.00 moles of D?arrow_forward
- [Review Topics] [References] "Smelling salts," which are used to revive someone who has fainted, typically contain ammonium carbonate, (NH4)2CO3. Ammonium carbonate decomposes readily to form ammonia, carbon dioxide, and water. The strong odor of the ammonia usually restores consciousness in the person who has fainted. The unbalanced equation is (NH4)2CO3 (8)→ NH3(g) + CO₂(g) + H₂O(g) Calculate the mass of ammonia gas that is produced if 0.850 g of ammonium carbonate decomposes completely. g NH3 Submit Answer Mastered Retry Entire Group 8 more group attempts remaining Previous Next Save and Exitarrow_forwardWhich reaction has the largest AS? ON₂(g) + 3H₂(g) → 2NH3(9) □2NO(g) → N₂O2(g) O2N₂ H₁(g) →→→ 2NH3(g) + H₂(g) □ O₂(g) + 2H₂(g) → 2H₂O(g) □ O2(g) +2H₂(g) → 2H₂O(l)arrow_forwardDoes the product formed during the combustion of magnesium in Test 6 (the ashy magnesium oxide) to weigh more than, less than, or the same as the original piece of magnesium? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY