
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
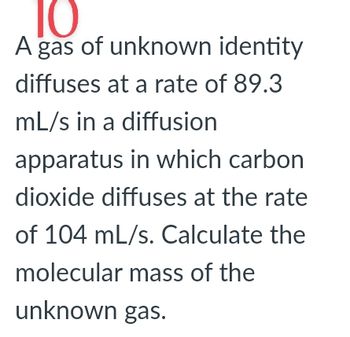
Transcribed Image Text:10
A gas of unknown identity
diffuses at a rate of 89.3
mL/s in a diffusion
apparatus in which carbon
dioxide diffuses at the rate
of 104 mL/s. Calculate the
molecular mass of the
unknown gas.

Transcribed Image Text:balloon filled with helium
gas takes 8.73 hours to
deflate to 78.3% of its
original volume. How long
will it take for an identical
balloon filled with the same
volume of hydrogen gas
(instead of helium) to
decrease its volume by
78.3%?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At a particular pressure and temperature, nitrogen gas effuses at the rate of 79 mL/s. Using the same apparatus at the same temperature and pressure, at what rate will sulfur dioxide effuse?arrow_forwardA 1.56 mol sample of O2 gas is confined in a 37.6 liter container at 20.9 °C. If 1.56 mol of H2 gas is added and the volume and temperature of the system are held constant, explain in at least one sentence what will happen to... a. The average molecular speed of the system. In your answer, you should indicate whether the average molecular speed will increase, decrease or remain the same, and also indicate your reasoning why. b. The average kinetic energy of the system. In your answer, you should indicate whether the average kinetic energy will increase, decrease or remain the same, and also indicate your reasoning why.arrow_forwardImagine that unknown gas in the storage tank is analyzed. A 14.74-g sample of the gas contains 9.97 g N2, 1.39 g CH4 , and 2.65 g CO2. Calculate the partial pressures of each gas (in bar) in the storage tank if the total pressure in the tank is 3.15 bar.arrow_forward
- https://youtu.be/TM3YiYajGf4?si=v8GatfHVrWh6zo02 In an observation table record the relative volumes of gas in the three sealed bags. Is there evidence that the amount of product formed is related to the amount of reactants used? Is there evidence that some of the baking soda has not completely reacted in one or more of the bags? Open each bag and add 15mL of vinegar to each. Is there evidence that a shortage of vinegar was limiting the production of carbon dioxide gas in some bags? Add 15mL of baking soda to each bag. Is there evidence that a lack of baking soda was limiting the production of carbon dioxide in some bags?arrow_forwardThe rate of effusion of a particular gas was measured and found to be 24.0mL/min. Under the same conditions the rate of effusion of pure methane( CH4) has is 47.8mL/min. What is the molar mass of the unknown gasarrow_forwardTo determine the molecular weight of an unknown chlorate. a sample of the chlorate was decomposed by heating and the oxygen gas produced was collected over water.563mL of oxygen were collected. The gas collected and the water were both at a temperature of 24°C. The total pressure of the room was 0.996 atm. If the vapor pressure of water at 24°C is 25.8 torr, howmany moles of gas were collected?R = 0.08206* L*atm/ mol*Karrow_forward
- A sample of O2 gas is observed to effuse through a porous barrier in 7.64 minutes. Under the same conditions, the same number of moles of an unknown gas requires 12.7 minutes to effuse through the same barrier. The molar mass of the unknown gas is g/mol.arrow_forwardA gas of unknown molecular mass was allowed to effuse through a small opening under constant pressure conditions. It required 72 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 27 s for 1.0 L of 02 gas to effuse. Calculate the molar mass of the unknown gas. (Remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 L; that is, rate and time are inversely proportional.) 367.1175859 X g/molarrow_forwardMI < CO C. Under identical conditions, separate samples of O2 and an unknown gas were allowed to effuse through identical membranes simultaneously. After a certain amount of time, it was found that 5.75 mL of O2 had passed through the membrane, but only 3.86 mL of of the unknown gas had passed through. What is the molar mass of the unknown gas? unknown molar mass: g/mol MacBook Pro The %23 %$4 delet 4. 5. 3. R. P. H. K. B. MOSISO command uondoarrow_forward
- A sample of SO2 gas is observed to effuse through a porous barrier in 5.45 minutes. Under the same conditions, the same number of moles of an unknown gas requires 4.27 minutes to effuse through the same barrier. The molar mass of the unknown gas is g/mol.arrow_forwardA sample of O3 gas is observed to effuse through a pourous barrier in 1.06 minutes. Under the same conditions, the same number of moles of an unknown gas requires 0.736 minutes to effuse through the same barrier. The molar mass of the unknown gas is g/mol.arrow_forwardMatch the characteristic of gases described below to the postulates of the kinetic molecular theory that best explain that characteristic. (Note: You may need to list more than one postulate.) The pressure of a gas in a fixed volume increases when its temperature increases. Select one or more: The average kinetic energies of gas particles increase with an increase in temperature. Gas particles are widely spaced. A gas consists of many small particles in rapid, random motion. The total volume of the molecules themselves is very small compared to the volume of the container. There are virtually no attractive forces between gas particles.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY