
Chemistry: An Atoms First Approach
2nd Edition
ISBN: 9781305079243
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I been having a hard time solving this example problem.
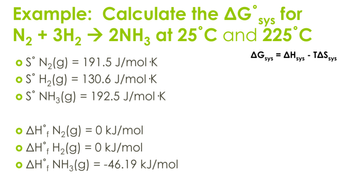
Transcribed Image Text:sys
Example: Calculate the AG˚ve for
N₂+ 3H2 → 2NH3 at 25°C and 225°C
S° N2(g) 191.5 J/mol K
=
S° H2(g) = 130.6 J/mol K
=
S° NH3(g) 192.5 J/mol K
o AH, N2(g) = 0 kJ/mol
• AH H2(g) = 0 kJ/mol
• AH NH3(g) = -46.19 kJ/mol
=
AG sys AH sys - TAS sys
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Would the amount of heat absorbed by the dissolution in Example 5.6 appear greater, lesser, or remain the same if the heat capacity of the calorimeter were taken into account? Explain your answer.arrow_forwardThe thermochemical equation for the burning of methane, the main component of natural gas, is CH4(g)+2O2(g)CO2(g)+2H2O(l)H=890kJ (a) Is this reaction endothermic or exothermic? (b) What quantities of reactants and products are assumed if H = 890 kJ? (c) What is the enthalpy change when 1.00 g methane burns in an excess of oxygen?arrow_forwardCalculate H for the reaction N2H4(l)+O2(g)N2(g)+2H2O(l) given the following data: Equation H(KJ) 2NH3(g)+3N2O(g)4N2(g)+3H2O(l) 1010 N2O(g)+3H2(g)N2H4(l)+H2O(l) 317 2NH3(g)+12O2(g)N2H4(l)+H2O(l) 143 H2(g)+12O2(g)H2O(l) 286arrow_forward
- Given: 2Cu2O(s) + O2(g) 4CuO(s)H = 288 kJ Cu2O(s) CuO(s) + CuO(s)H = 11kJ Calculate the standard enthalpy of formation (Ht) for CuO(s).arrow_forwardIn a coffee-cup calorimeter, 150.0 mL of 0.50 M HCI is added to 50.0 mL of 1.00 M NaOH to make 200.0 g solution at an initial temperature of 48.2C. If the enthalpy of neutralization for the reaction between a strong acid and a strong base is 56 kJ/mol, calculate the final temperature of the calorimeter contents. Assume the specific heat capacity of the solution is 4.184 J/g C and assume no heat Joss to the surroundings.arrow_forwardGive the definition of the standard enthalpy of formation for a substance. Write separate reactions for the formation of NaCl, H2O , C6H12O6, and PbSO4 that have H values equal to Hf for each compound.arrow_forward
- An industrial process for manufacturing sulfuric acid, H2SO4, uses hydrogen sulfide, H2S, from the purification of natural gas. In the first step of this process, the hydrogen sulfide is burned to obtain sulfur dioxide, SO2. 2H2S(g)+3O2(g)2H2O(l)+2SO2(g);H=1124kJ The density of sulfur dioxide at 25C and 1.00 atm is 2.62 g/L, and the molar heat capacity is 30.2 J/(mol C). (a) How much heat would be evolved in producing 1.00 L of SO2 at 25C and 1.00 atm? (b) Suppose heat from this reaction is used to heat 1.00 L of the SO2 from 25C to 500C for its use in the next step of the process. What percentage of the heat evolved is required for this?arrow_forwardGas A2 reacts with gas B2 to form gas AB at a constant temperature. The bond energy of AB is much greater than that of either reactant. What can be said about the sign of H? SSurr? S? Explain how potential energy changes for this process. Explain how random kinetic energy changes during the process.arrow_forwardDissolving 6.00 g CaCl2 in 300 mL of water causes the temperature of the solution to increase by 3.43 C. Assume that the specific heat of the solution is 4.18 J/g K and its mass is 306 g. (a) Calculate the enthalpy change when the CaCl2 dissolves. Is the process exothermic or endothermic? (b) Determine H on a molar basis for CaCl2(s)H2OCa2+(aq)+2Cl(aq)arrow_forward
- At 298 K, the standard enthalpies of formation for C2H2(g) and C6H6(l) are 227 kJ/mol and 49 kJ/mol, respectively. a. Calculate H for C6H6(l)3C2H2(g) b. Both acetylene (C2H2) and benzene (C6H6) can be used as fuels. Which compound would liberate more energy per gram when combusted in air?arrow_forwardA substance, X, has the following properties: Specific Heat Capacities Hvap 20.kj/mol C(s) 3.0j/gC Hfus 5.0kj/mol C(l) 2.5j/gC bp 75C C(g) 2.5j/gC mp 15Carrow_forwardThe process of dissolving ammonium nitrate, NH4NO3, in water is an endothermic process. What is the sign of q? If you were to add some ammonium nitrate to water in a flask, would you expect the flask to feel warm or cool?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning